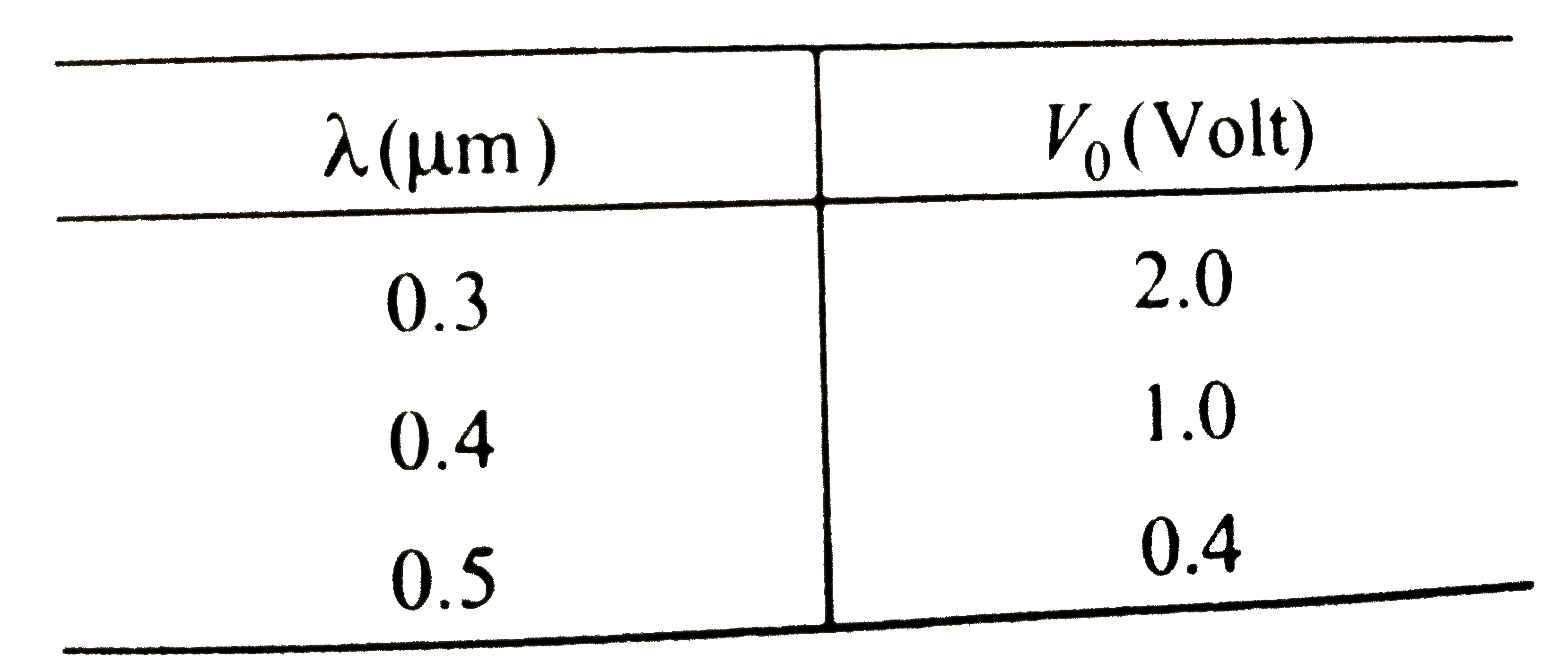
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Assertion Reason|22 VideosATOMIC STRUCTURE
OP TANDON|Exercise Matrix matching Type Question|5 VideosATOMIC STRUCTURE
OP TANDON|Exercise Set-2|46 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Objective Question Level-B
- The energy required for removal of electron fro 3s,3p,3d subshells of ...
Text Solution
|
- Which of the following electronic configuration have the highest excha...
Text Solution
|
- In a historical experiment to dtermine Planck's constant, a metal surf...
Text Solution
|
- Angular distribution functions of all orbitals have:
Text Solution
|
- If uncertainty in position and momentum are equal then uncertainty in ...
Text Solution
|
- The minimum number of waves made by an electron moving in an orbit hav...
Text Solution
|
- The number of elliptical orbits excluding circular orbits in the N-she...
Text Solution
|
- From the electronic configuration of the given elements K,L, M and N, ...
Text Solution
|
- Radiation of wavelength lambda in indent on a photocell . The fastest ...
Text Solution
|
- Given set of quantum numbers for a multielectron atom is: {:(n,l,m,s...
Text Solution
|
- In how many elements does the electron have the quantum number of n =...
Text Solution
|
- If there are three possible values (-1//2,0,+1//2) for the spin quantu...
Text Solution
|
- The radius of first Bohr orbit is x, then de-Broglie wavelength of ele...
Text Solution
|
- How many times does light travel faster in vacuum than an electron in ...
Text Solution
|
- A compound of vanadium has a magnetic moment of 1.73 BM. The electroni...
Text Solution
|
- The angular momentum of an electron revolving in a p-orbital is
Text Solution
|
- If a hydrogen atom emit a photon of energy 12.1 eV , its orbital angul...
Text Solution
|
- The total energy of the electron of H-atom in the second quantum state...
Text Solution
|
- What is the ratio of the Rydberg constant for helium to Hydrogen atom?
Text Solution
|
- If the kinetic energy of a particle is doubled, de Broglie wavelength ...
Text Solution
|