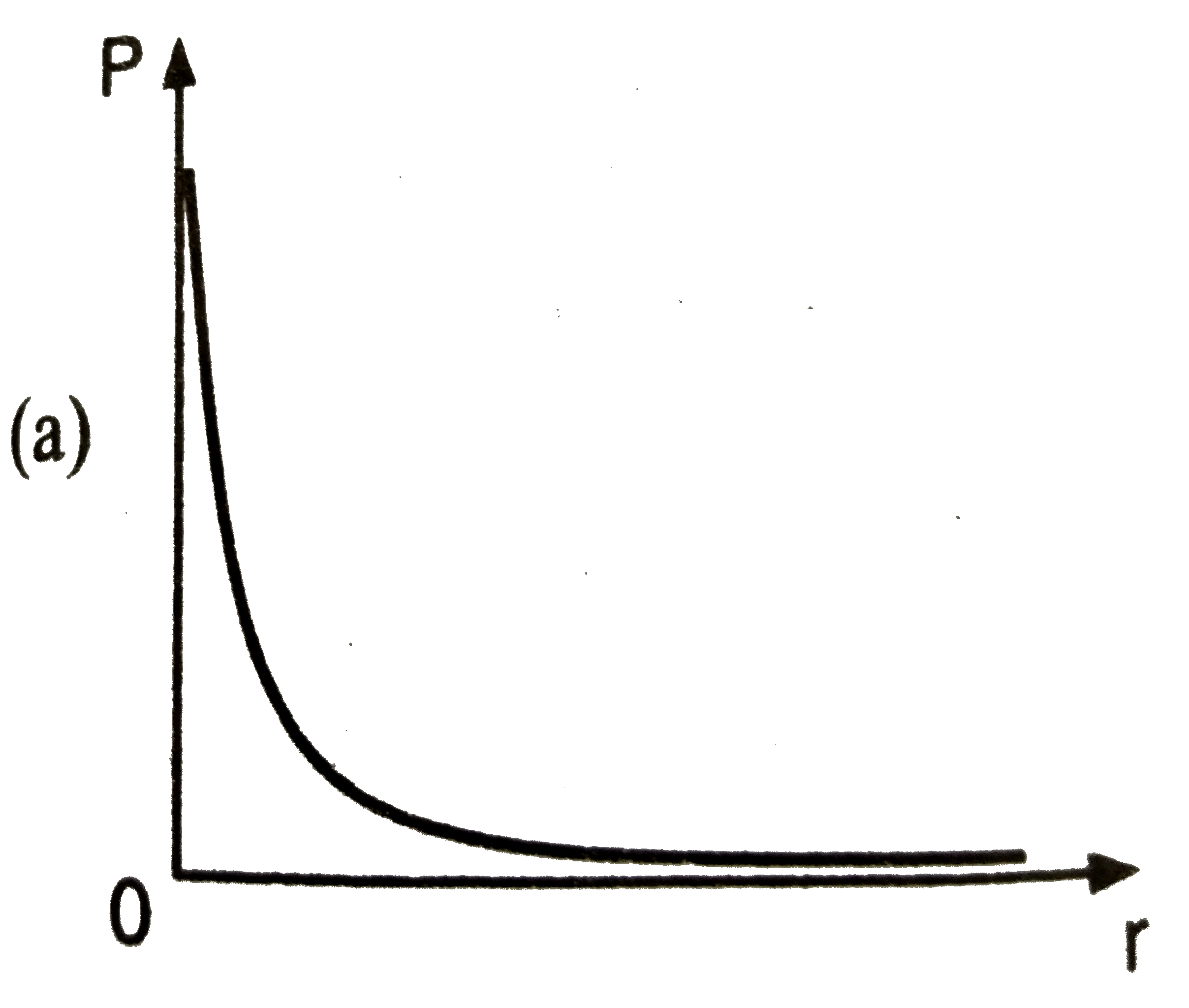
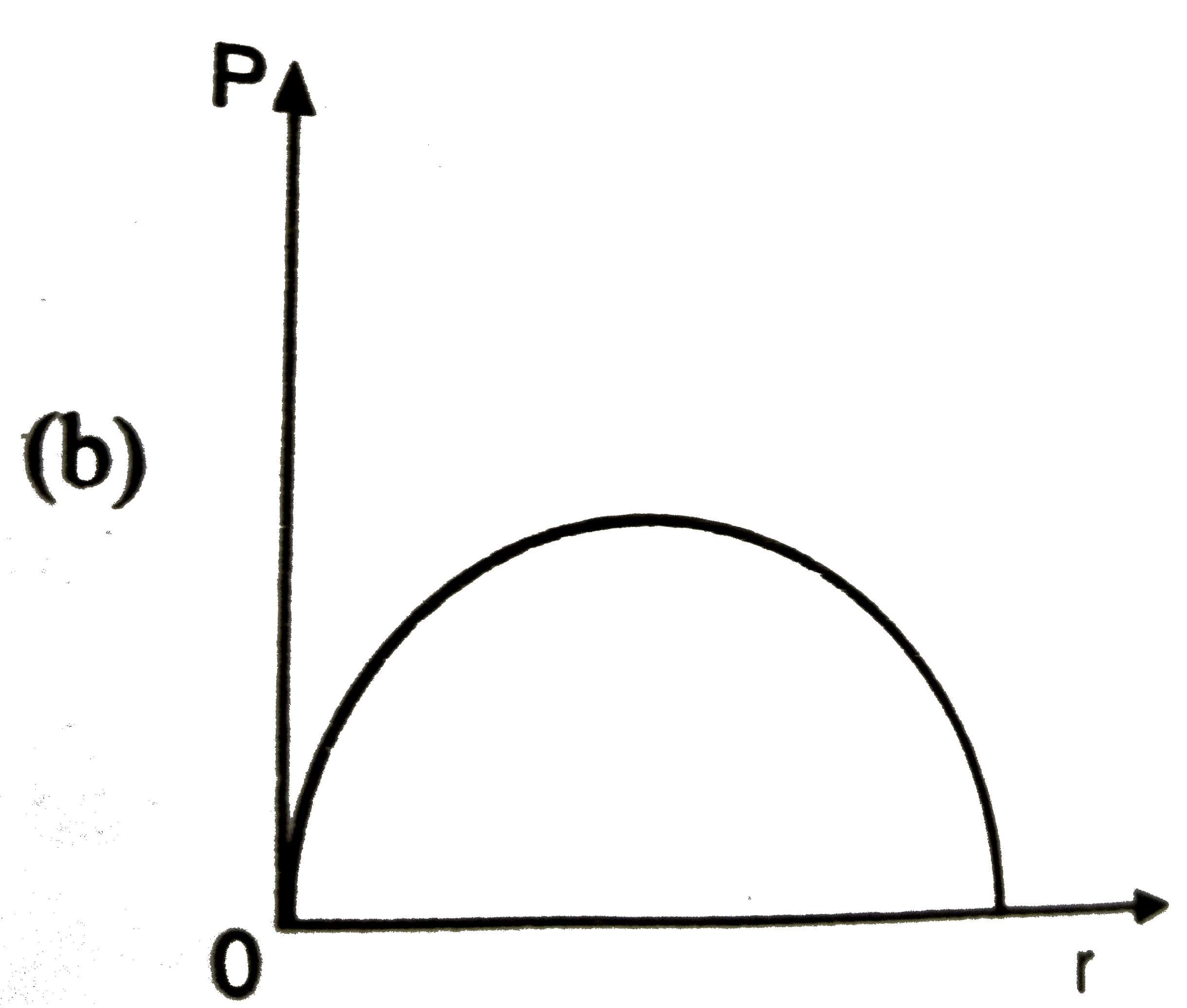
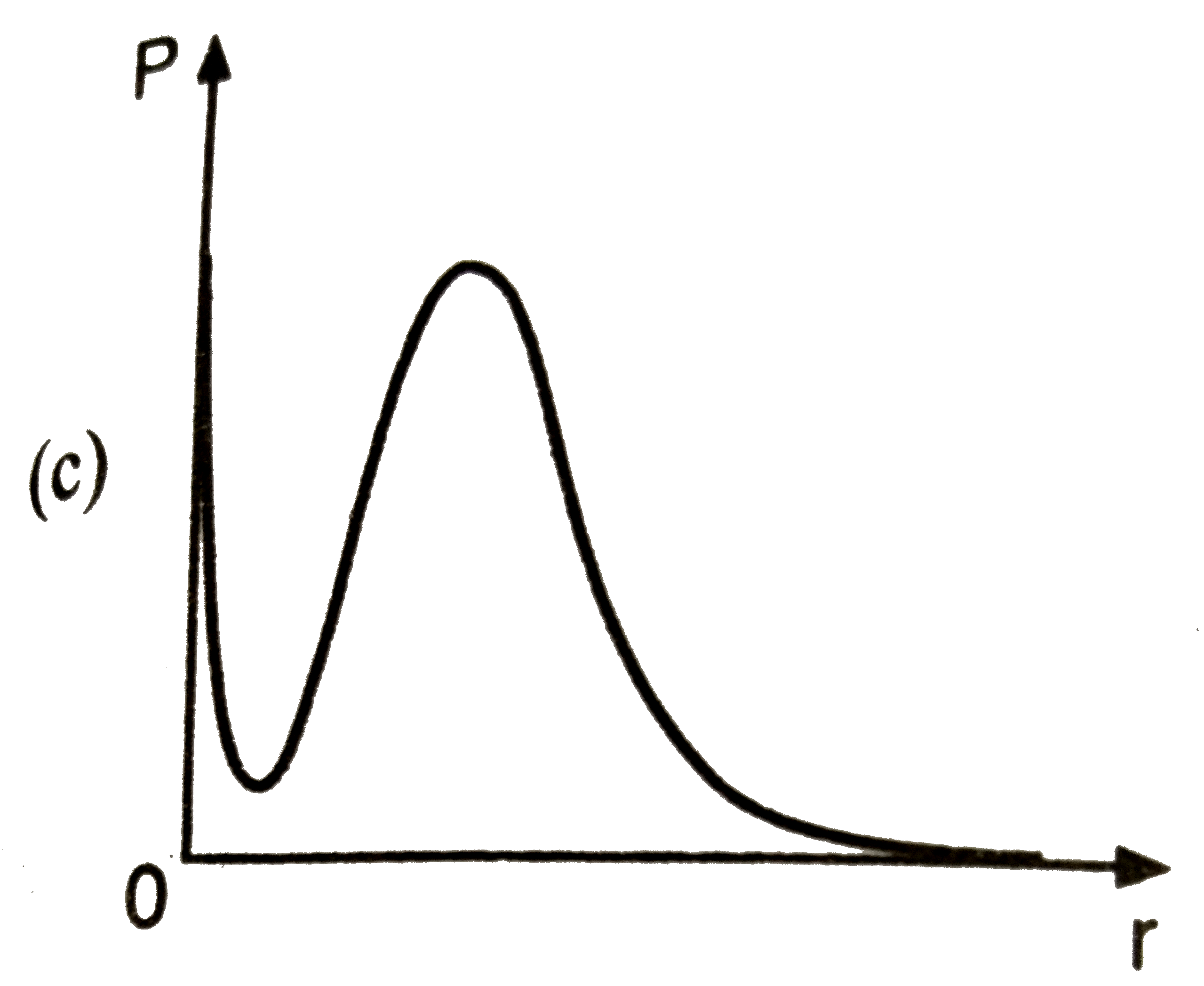
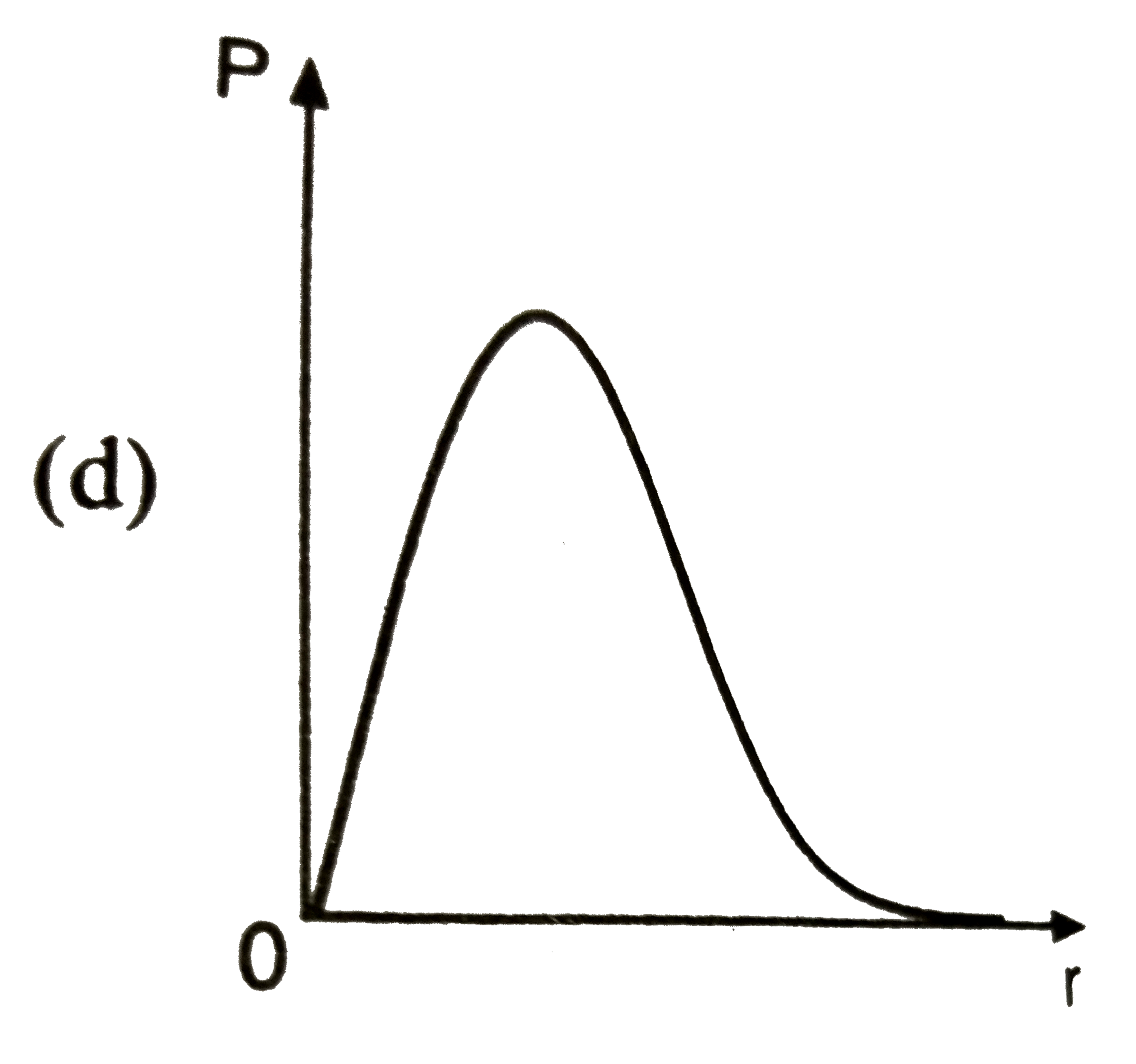
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Assertion Reason|22 VideosATOMIC STRUCTURE
OP TANDON|Exercise Matrix matching Type Question|5 VideosATOMIC STRUCTURE
OP TANDON|Exercise Set-2|46 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Objective Question Level-B
- The angular momentum of an electron revolving in a p-orbital is
Text Solution
|
- If a hydrogen atom emit a photon of energy 12.1 eV , its orbital angul...
Text Solution
|
- The total energy of the electron of H-atom in the second quantum state...
Text Solution
|
- What is the ratio of the Rydberg constant for helium to Hydrogen atom?
Text Solution
|
- If the kinetic energy of a particle is doubled, de Broglie wavelength ...
Text Solution
|
- Imagine an atom made of a proton and a hypothetical particle of double...
Text Solution
|
- What is the ratio of time periods (T(1)//T(2)) in second orbit of hydr...
Text Solution
|
- The de Broglie wavelength of an electron accelerated by an electric fi...
Text Solution
|
- An excited state of H atom emits a photon of wavelength lamda and retu...
Text Solution
|
- A dye absorbs a photon of wavelength lamda and re-emits the same energ...
Text Solution
|
- A metal is irradiated with light of wavelength 660 nm. Given that the ...
Text Solution
|
- Hydrogen ((1)H^(1)) Deuterium ((1)H^(2)) singly omised helium ((1)He...
Text Solution
|
- If the radius of 3rd orbit of hydrogen atom is 'x' then the radius of ...
Text Solution
|
- Which of the following graphs between radial probability distribution ...
Text Solution
|
- An electron in a hydrogen atom in its ground state absorbs energy equa...
Text Solution
|
- Which orbital has only positive value of wave function at all distance...
Text Solution
|
- A metal is irradiated with light of frequency 3.2xx10^(16)Hz. The ejec...
Text Solution
|
- Spin angular momentum of nitrogena to in ground state is:
Text Solution
|
- A plot of kinetic energy of the emitted electron against frequency of...
Text Solution
|
- P is the probability of finding the Is electron of hydrogen atom in a ...
Text Solution
|