Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
OP TANDON|Exercise Linked Comprehension Type Question|49 VideosATOMIC STRUCTURE
OP TANDON|Exercise Self Assement|20 VideosATOMIC STRUCTURE
OP TANDON|Exercise Matrix matching Type Question|5 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 VideosCHARACTERISATION OF ORGANIC COMPOUNDS
OP TANDON|Exercise Passage-2|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ATOMIC STRUCTURE -Integer Type Question
- For Li^(2+), when an electron falls from a higher orbit to nth orbit, ...
Text Solution
|
- The emission lines of hydrogen contains ten lines. The highest orbit i...
Text Solution
|
- Total number of nodes present in 4d-orbitals will be:
Text Solution
|
- Spin multiplicity of Nitrogen atom is
Text Solution
|
- Orbital frequency of electron in nth orbit of hydrogen is twice that o...
Text Solution
|
- If kinetic energy of an electron is reduced by (1/9) then how many tim...
Text Solution
|
- If electrons in hydrogen sample return from 7th shell to 4 th shell th...
Text Solution
|
- An electron in Li^(2+) ion is in excited state (n(2)). The wavelength ...
Text Solution
|
- The energy corresponding to one of the lines in the paschen series of ...
Text Solution
|
- The angular momentum of electron in the shell in which the g-subshell ...
Text Solution
|
- The maximum number of electrons that can have principal quantum number...
Text Solution
|
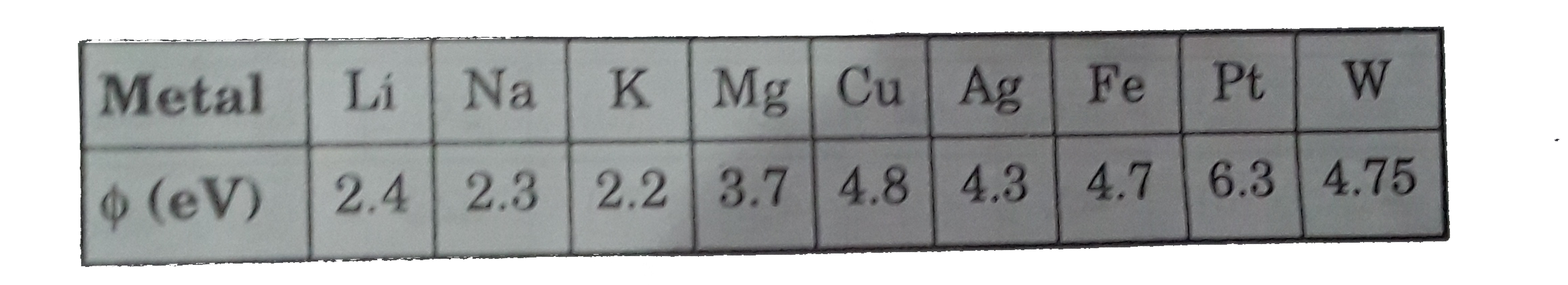
- The work function (phi) of some metals is listed below . The number of...
Text Solution
|
- The work function of Silver and sodium are 4.6 and 2.3 eV, respective...
Text Solution
|
- The atomic masses of He and Ne are 4 and 20 amu respectively . The va...
Text Solution
|
- In an atom, the total number of electrons having quantum numbers n =...
Text Solution
|
- Not considering the electronic spin, the degeneracy of the second exci...
Text Solution
|
- A hydrogen atom in its ground state is irradiated by light of waveleng...
Text Solution
|