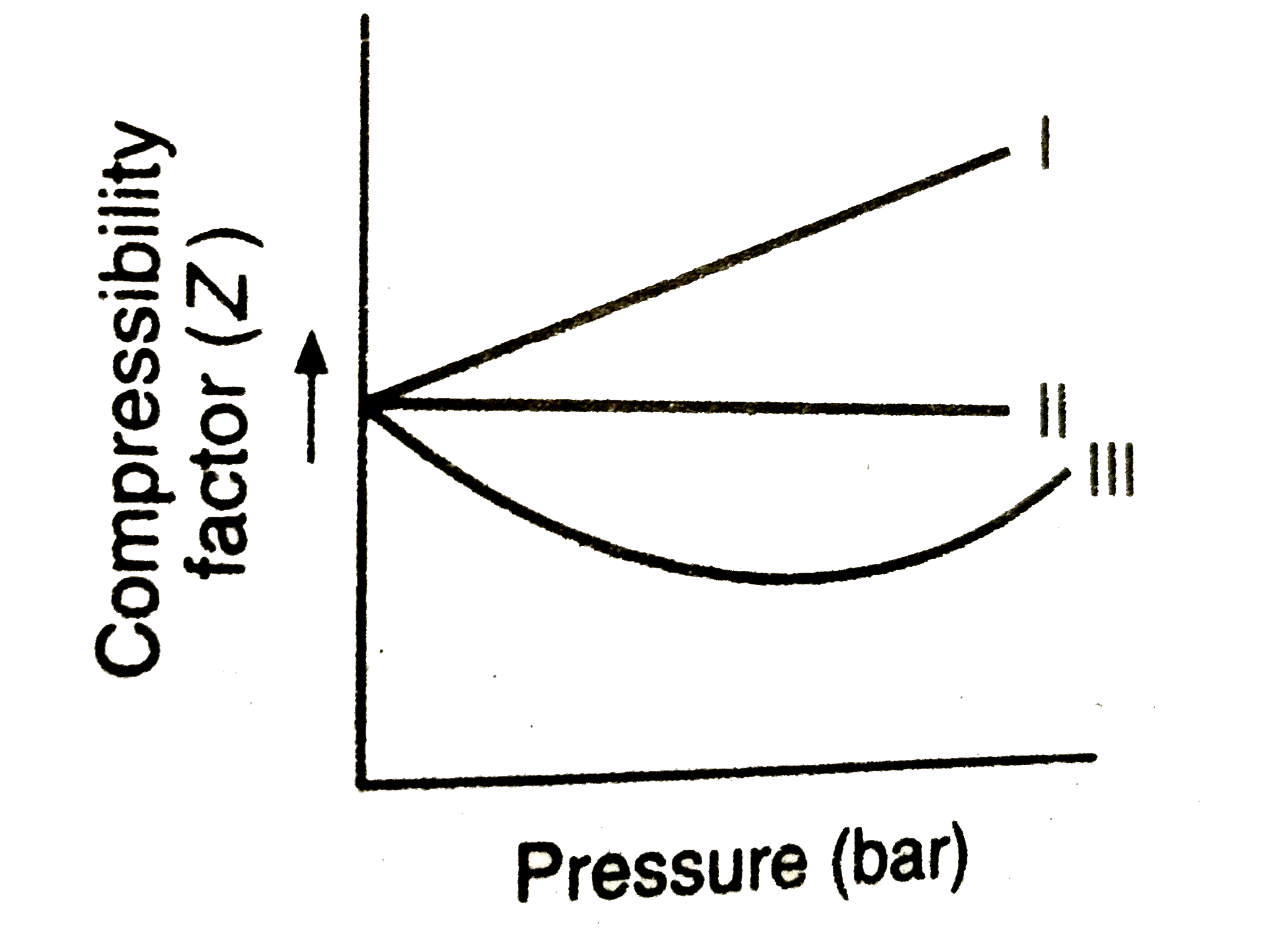
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Set 2|40 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions Level B|34 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Practice Problems|53 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Objective questions
- By what factor does the average velocity of a gaseous molecule increas...
Text Solution
|
- The van der Waals' constant 'a' for the gases O(2), N(2), NH(3) "and "...
Text Solution
|
- The Boyle temperature of three gases are given in the table : {:("Ga...
Text Solution
|
- Joule-Thomson coefficient is zero at:
Text Solution
|
- Two gases A and B having the same volume diffuse through a porous part...
Text Solution
|
- 5 g of unknown gas has pressure P at a temperature T K in a vessel. On...
Text Solution
|
- Which of the following expressions correctly represents the relationsh...
Text Solution
|
- Which of the following exhibits the weakest intermolecular forces?
Text Solution
|
- The compressibility of a gas is less than unity at STP, therefore,
Text Solution
|
- The compressibilty factor for a real gas at high pressure is:
Text Solution
|
- The rms velocity of hydrogen is sqrt7 times the rms velocity of nitrog...
Text Solution
|
- Kinetic energy of a molecule is zero at 0^(@)C
Text Solution
|
- Densities of two gases are in the ratio 1: 2 and their temperatures ar...
Text Solution
|
- For a fixed mass of an ideal gas the correct representation is:
Text Solution
|
- 4.4 g of a gas at STP occupies a volume of 2.24 L. The gas can be :
Text Solution
|
- At 0^(@)C and one atm pressure, a gas occupies 100 cc. If the pressure...
Text Solution
|
- Calculate the total pressure in a mixture of 4 g of oxygen and 2 g of ...
Text Solution
|
- Density ratio of O(2) " and " H(2) is 16 : 1. The ratio of their rms v...
Text Solution
|
- The rate of diffusion of a gas having molecular weight just double of ...
Text Solution
|
- The density of air is 0.00130 g/mL. The vapour density of air will be ...
Text Solution
|