A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Set 2|40 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions Level B|34 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Practice Problems|53 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Objective questions
- Which of the following is not a property of liquid state ?
Text Solution
|
- A one litre glass bulb is evacuated and weighed. The weight is 500 g. ...
Text Solution
|
- Which one of the following statement is not true about the effect of a...
Text Solution
|
- A gas can be liquefied
Text Solution
|
- To what temperature must a neon gas sample be heated to double its pre...
Text Solution
|
- Which of the following liquids has the maximum surface tension at a gi...
Text Solution
|
- If a volume containing gas is compressed to half, how many moles of ga...
Text Solution
|
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- Equal masses of methane and oxygen are mixed in an empty container at ...
Text Solution
|
- a' and 'b' are van der Waals' constants for gases Chlorine is more eas...
Text Solution
|
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- The van der Waals' coefficient of the inert gases He, Ar and Xe are gi...
Text Solution
|
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- For gaseous state, if most probable speed is denoted by C^(**) average...
Text Solution
|
- If Z is a compressibility factor, van der Waals' equation at low press...
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
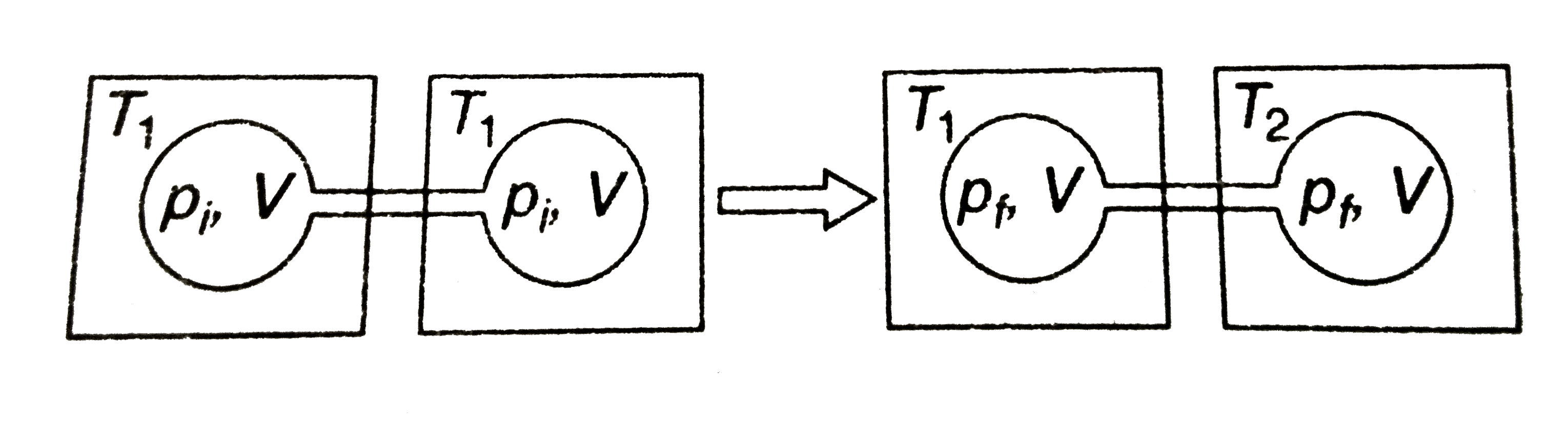
- Two closed bulbs of equal volume (V) containing an ideal gas initially...
Text Solution
|
- The process of real gases is less than the pressure of an ideal gas be...
Text Solution
|
- If the rms speed of nitrogen at a certain temperature is 3000 ms^(-1),...
Text Solution
|
- The compressibility factor for an ideal gas is
Text Solution
|