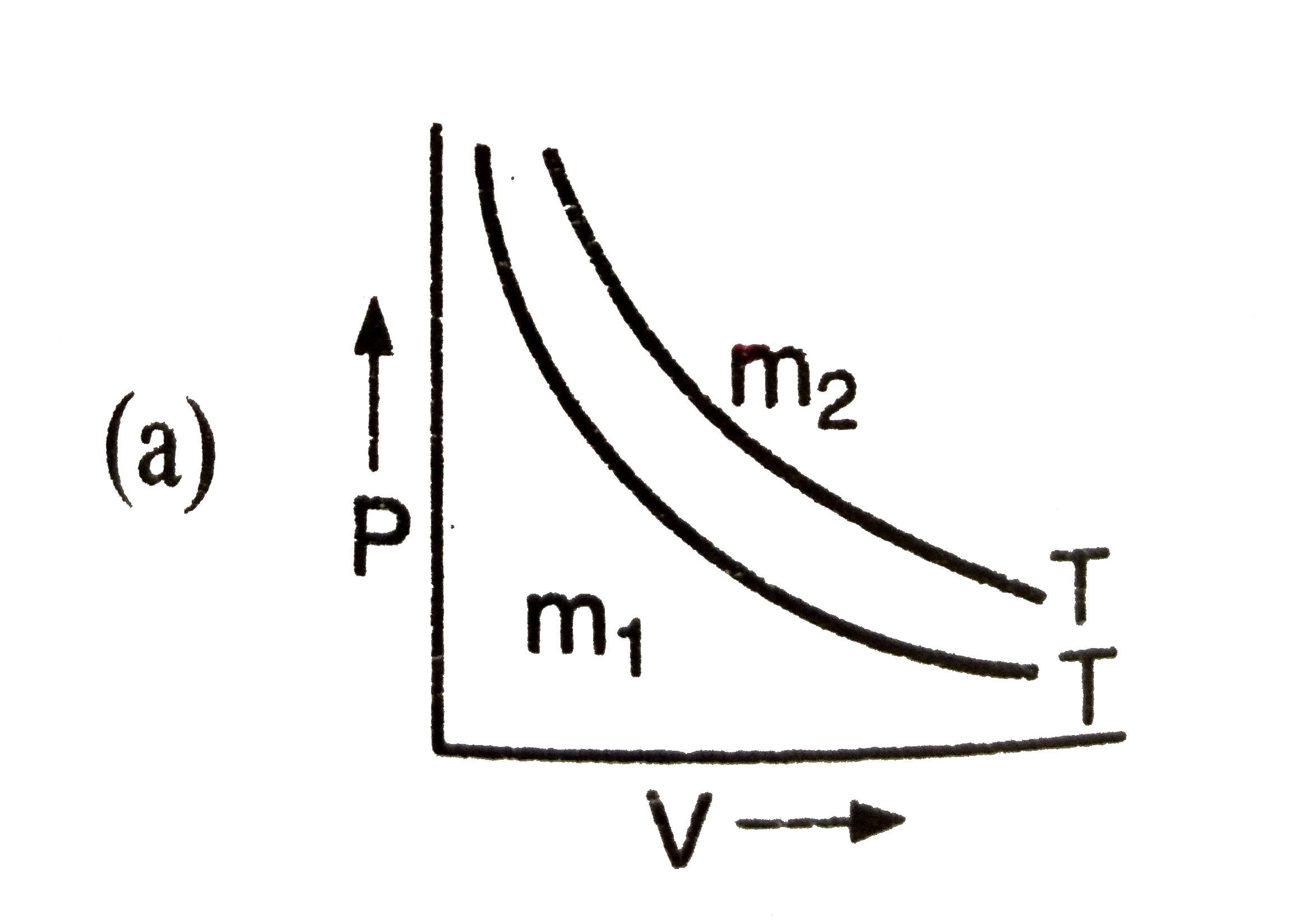
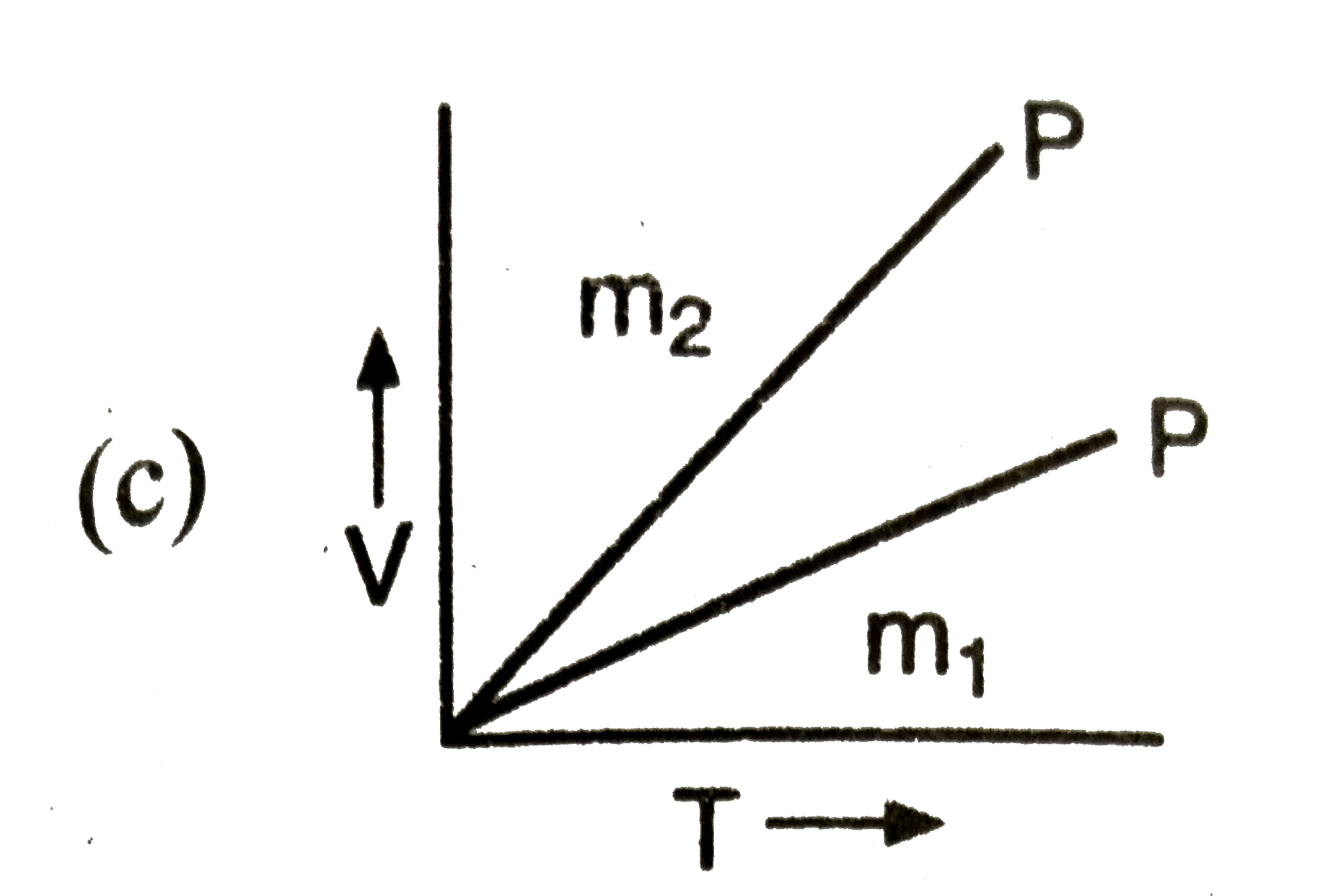
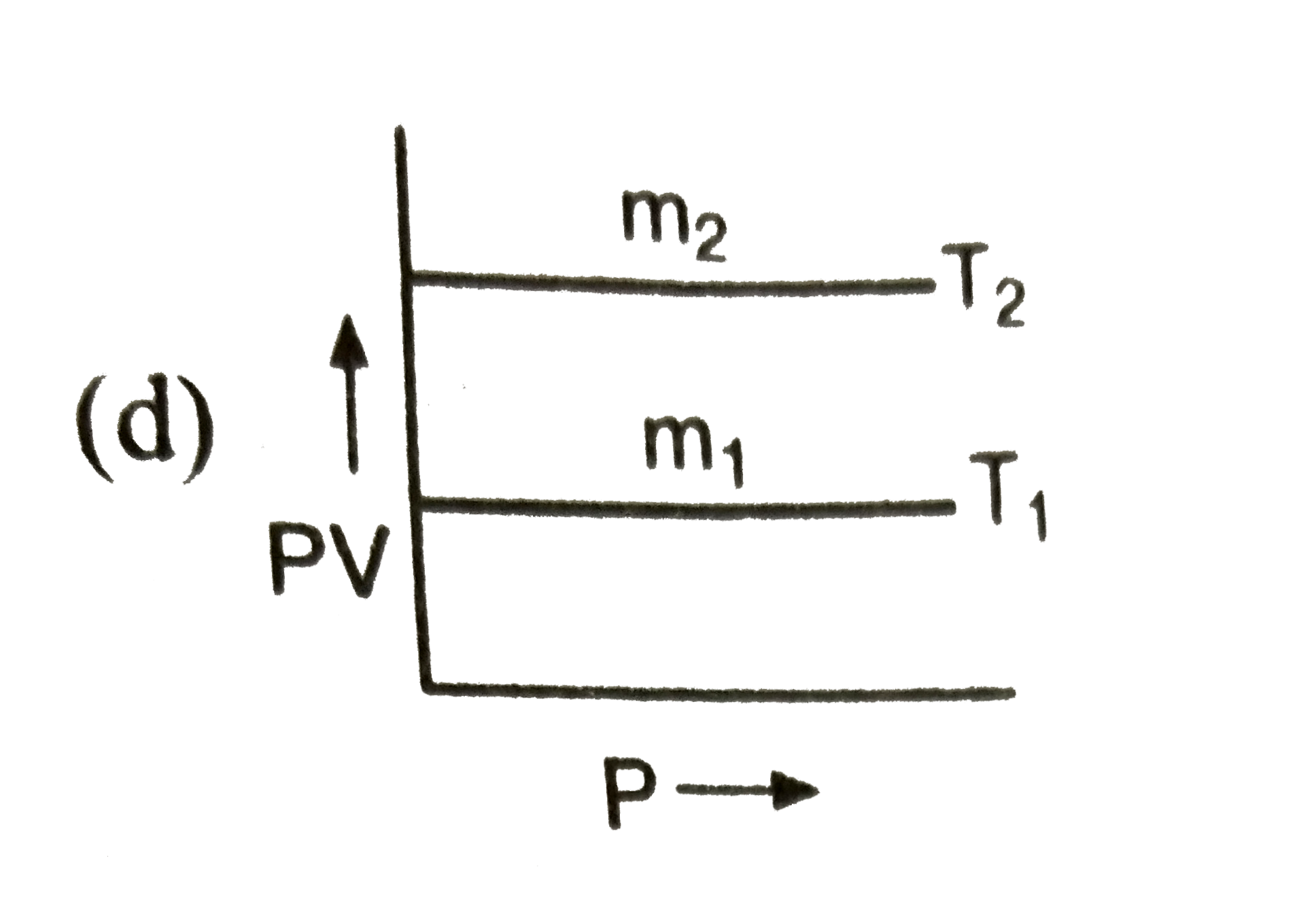
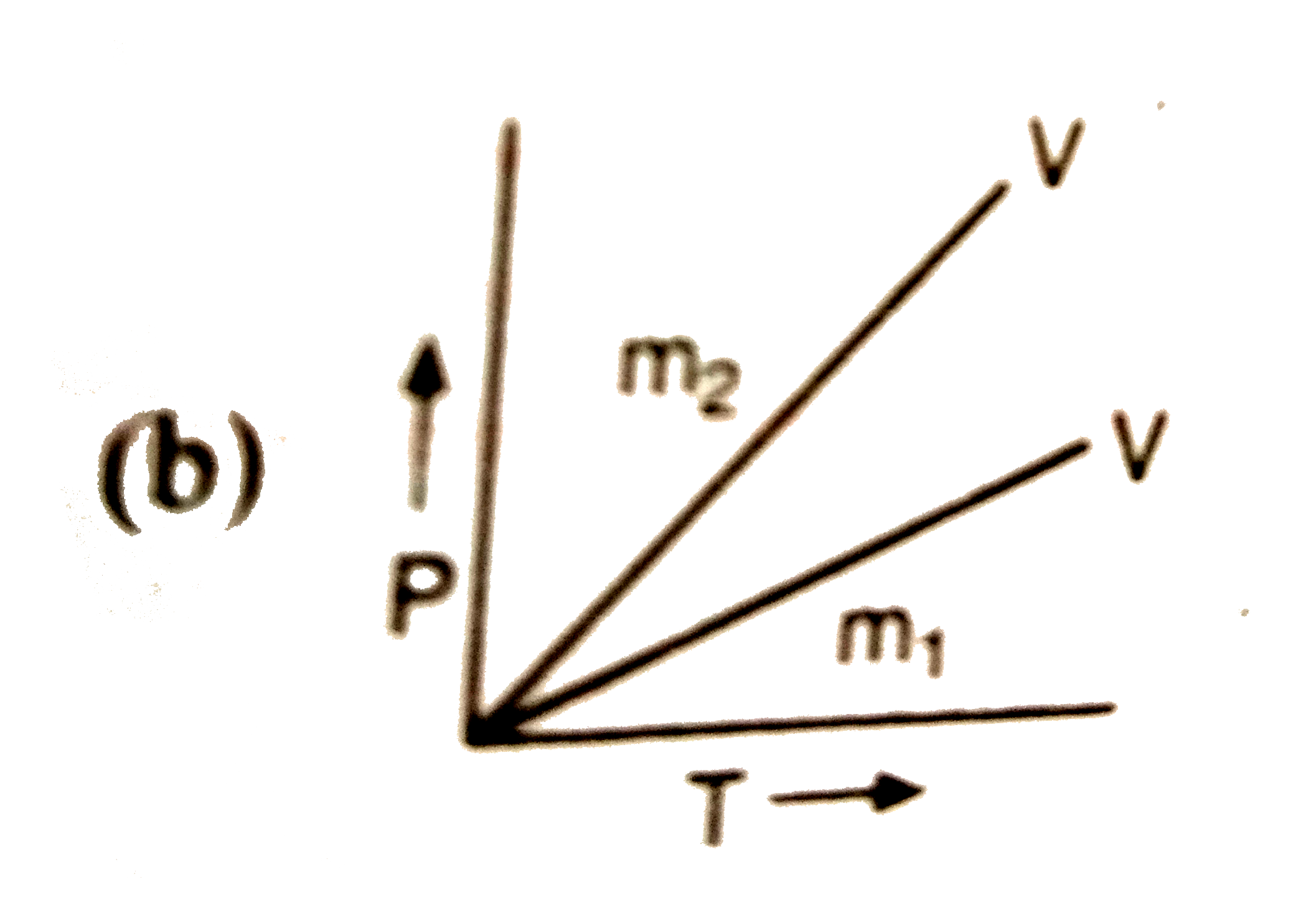A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions Level B|34 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Assertion-Reason|10 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions|105 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Set 2
- If force of attraction between the molecules is negligible, van der Wa...
Text Solution
|
- If m(1),m(2) are masses of an ideal gas, then which of the graph repre...
Text Solution
|
- van der Waals' constants for three different gases are given : {:("...
Text Solution
|
- What is the ratio of mean speed of an O(3) molecule to the rms speed o...
Text Solution
|
- Boyle's law may be expressed as
Text Solution
|
- If the pressure of a gas contained in a closed vessel is increased by ...
Text Solution
|
- The graph that does represent the behaviour of an ideal gas is :
Text Solution
|
- According to Charles' law :
Text Solution
|
- In the following statements : (A) Ideal gases are liquefied only at ...
Text Solution
|
- Which of the following relationships is/are not true ?
Text Solution
|
- According to kinetic theory of gases, for a datomic molecule.
Text Solution
|
- A gas described by van der Waals equation .
Text Solution
|
- According to kinetic theory of gases:
Text Solution
|
- Which of the following graph represent Boyle's law ?
Text Solution
|
- Which of the following equation (s) is/are correct on the basis of ide...
Text Solution
|
- Assertion: A gas can be easily liquefied at any temperature below is c...
Text Solution
|
- Which of the following quantities is the same for all ideal gases at t...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- The viscosity of a liquid molecule depends on :
Text Solution
|
- Viscosity si property of
Text Solution
|