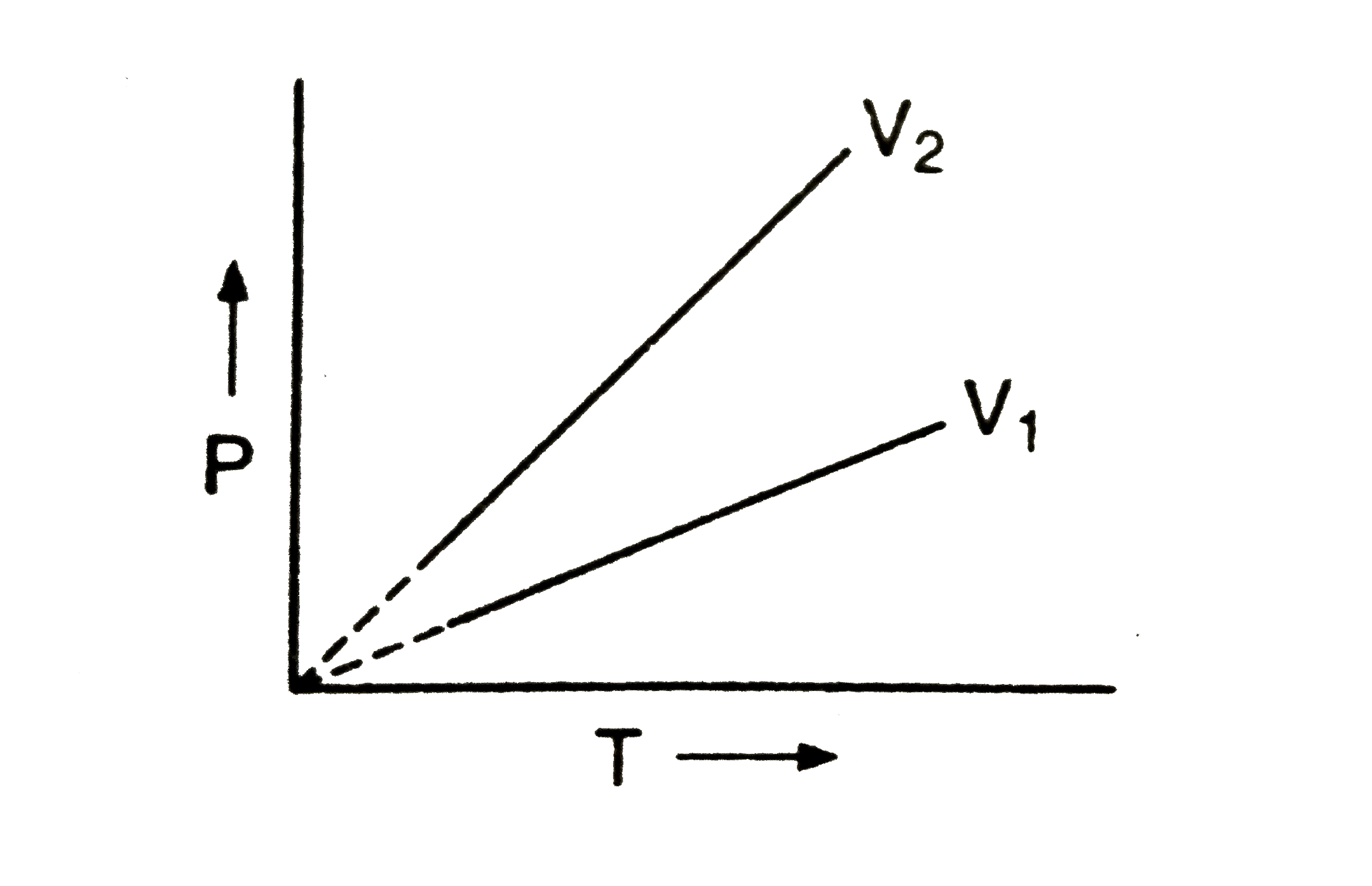
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Assertion-Reason|10 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Matrix Matching type questions|7 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Set 2|40 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Objective questions Level B
- In the corrections made to the ideal gas equation for real gases, the ...
Text Solution
|
- Which of the following is most suitable for liquefaction?
Text Solution
|
- An ideal gas of certain mass is heated in a small vessel and then in a...
Text Solution
|
- A spherical air bubble is rising from the depth of a lake when pressur...
Text Solution
|
- It is eaiser to liquefy oxygen than hydrogen because.
Text Solution
|
- 2 mol He is mixed with 2 gm of H(2). The molar heat capacity at const...
Text Solution
|
- The van der Waals' constant 'a' for the gases O(2), N(2), NH(3) "and "...
Text Solution
|
- At what temperature will the molar kinetic energy of 0.3 mol of 'He' b...
Text Solution
|
- Let P and P(s) be the partial pressure of water and saturated partial ...
Text Solution
|
- There are three closed containers in which equal moles of gas is fille...
Text Solution
|
- A vessel is filled with a mixture of oxygen and nitrogen. At what rati...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Which of following correctly represents the relation between capillary...
Text Solution
|
- There is a depression in the surface of the liquid in a capillary when
Text Solution
|
- Surface tension does not vary with
Text Solution
|
- Which among of the following has least surface tension?
Text Solution
|
- The SI unit of viscosity coefficient is
Text Solution
|
- Under critical conditions, the compressibility factor for a gas is .
Text Solution
|
- The critical temperature of water is higher than that of O(2) because ...
Text Solution
|
- The ratio a/b (the terms used in van der Waals' equation) has the unit...
Text Solution
|