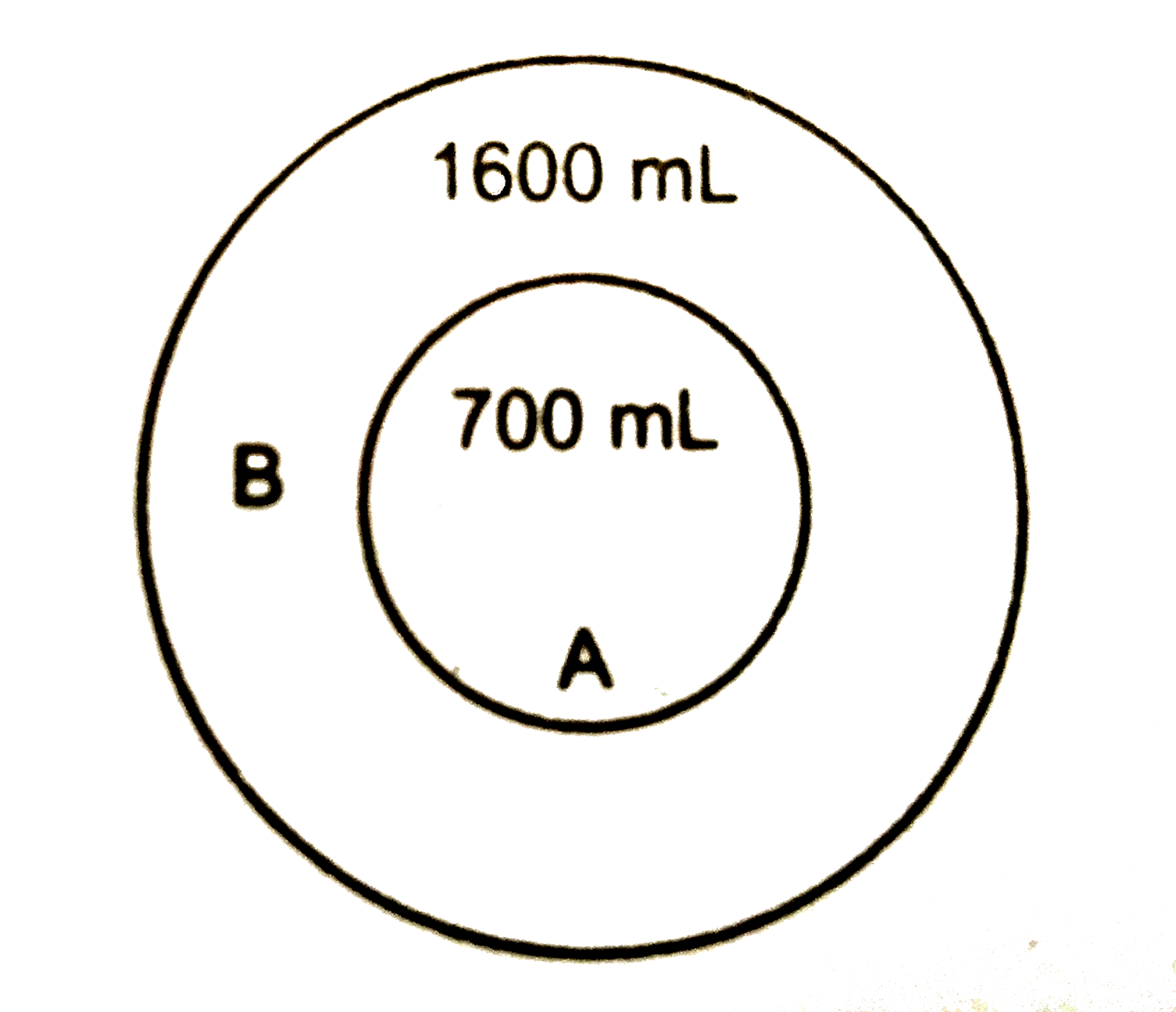A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Assertion-Reason|10 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Matrix Matching type questions|7 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Set 2|40 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Objective questions Level B
- There are three closed containers in which equal moles of gas is fille...
Text Solution
|
- A vessel is filled with a mixture of oxygen and nitrogen. At what rati...
Text Solution
|
- Which of the following is incorrect ?
Text Solution
|
- Which of following correctly represents the relation between capillary...
Text Solution
|
- There is a depression in the surface of the liquid in a capillary when
Text Solution
|
- Surface tension does not vary with
Text Solution
|
- Which among of the following has least surface tension?
Text Solution
|
- The SI unit of viscosity coefficient is
Text Solution
|
- Under critical conditions, the compressibility factor for a gas is .
Text Solution
|
- The critical temperature of water is higher than that of O(2) because ...
Text Solution
|
- The ratio a/b (the terms used in van der Waals' equation) has the unit...
Text Solution
|
- Select the correct order of the following temperature: {:("Boyl tempe....
Text Solution
|
- The gas equation for a real gas is: P (V - b) = RT Here, the param...
Text Solution
|
- Two gas bulbs are connected by a thin tube. Calaculate the partial pre...
Text Solution
|
- A manometer attached to a flask contains with ammonia gas have no diff...
Text Solution
|
- Two balloon A and B are taken at 300 K. Maximum capacity of balloon A ...
Text Solution
|
- A gas jar of 10 litre volume filled with O(2) at 300 K is connected to...
Text Solution
|
- Two non-reactive monoatomic ideal gases have their atomic masses in th...
Text Solution
|
- One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh...
Text Solution
|
- The qualitative sketches I,II and III given below show the variation ...
Text Solution
|