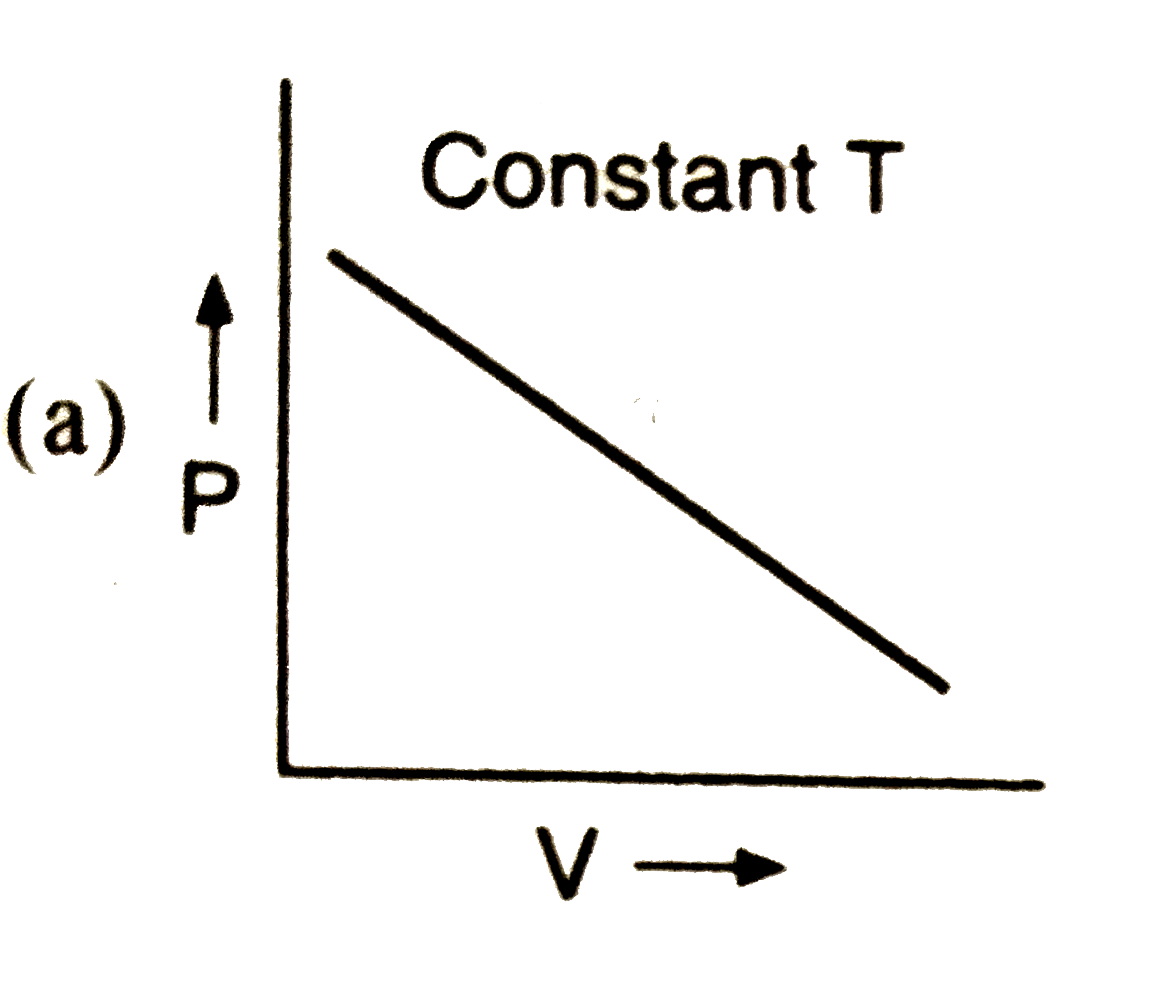
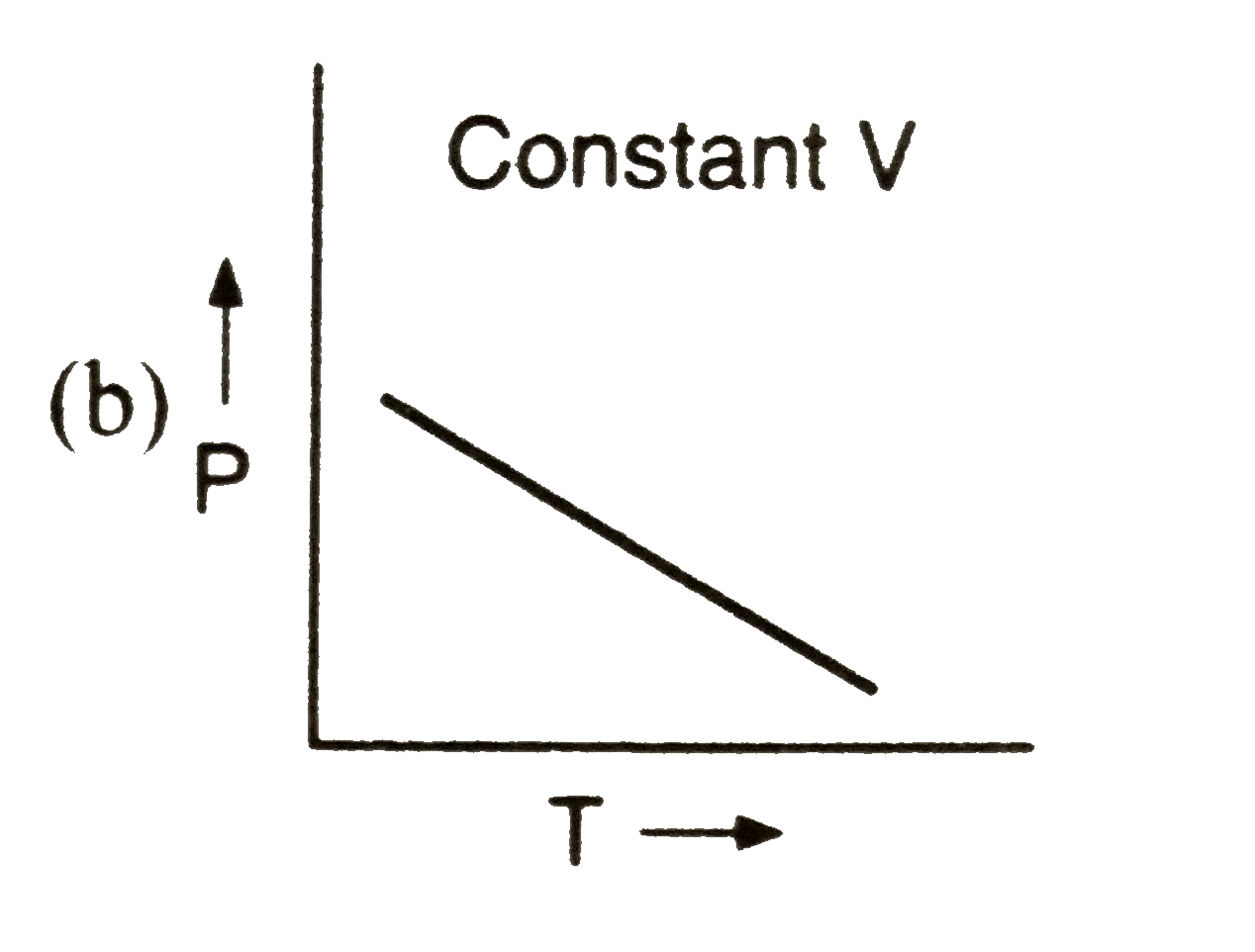
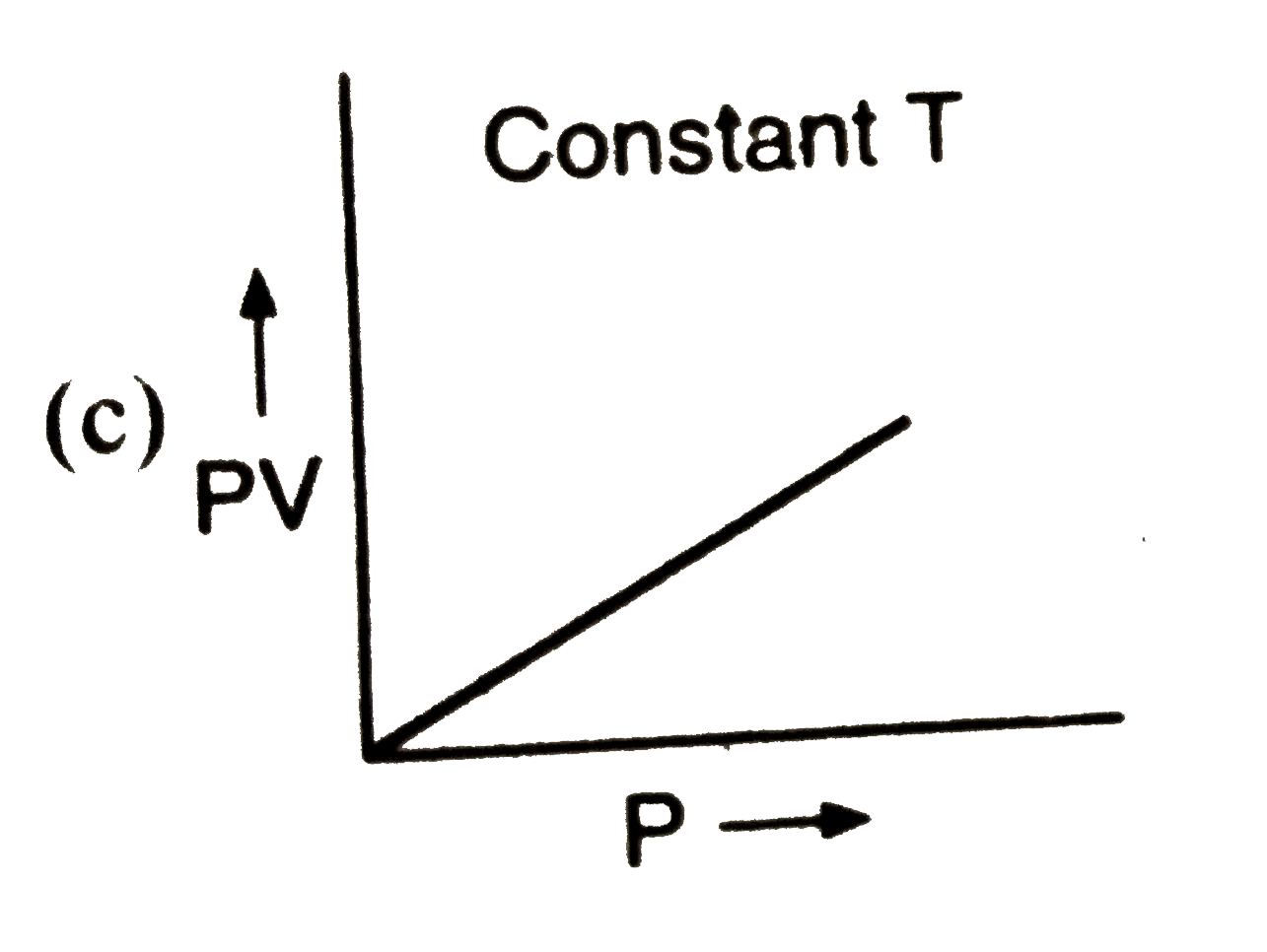
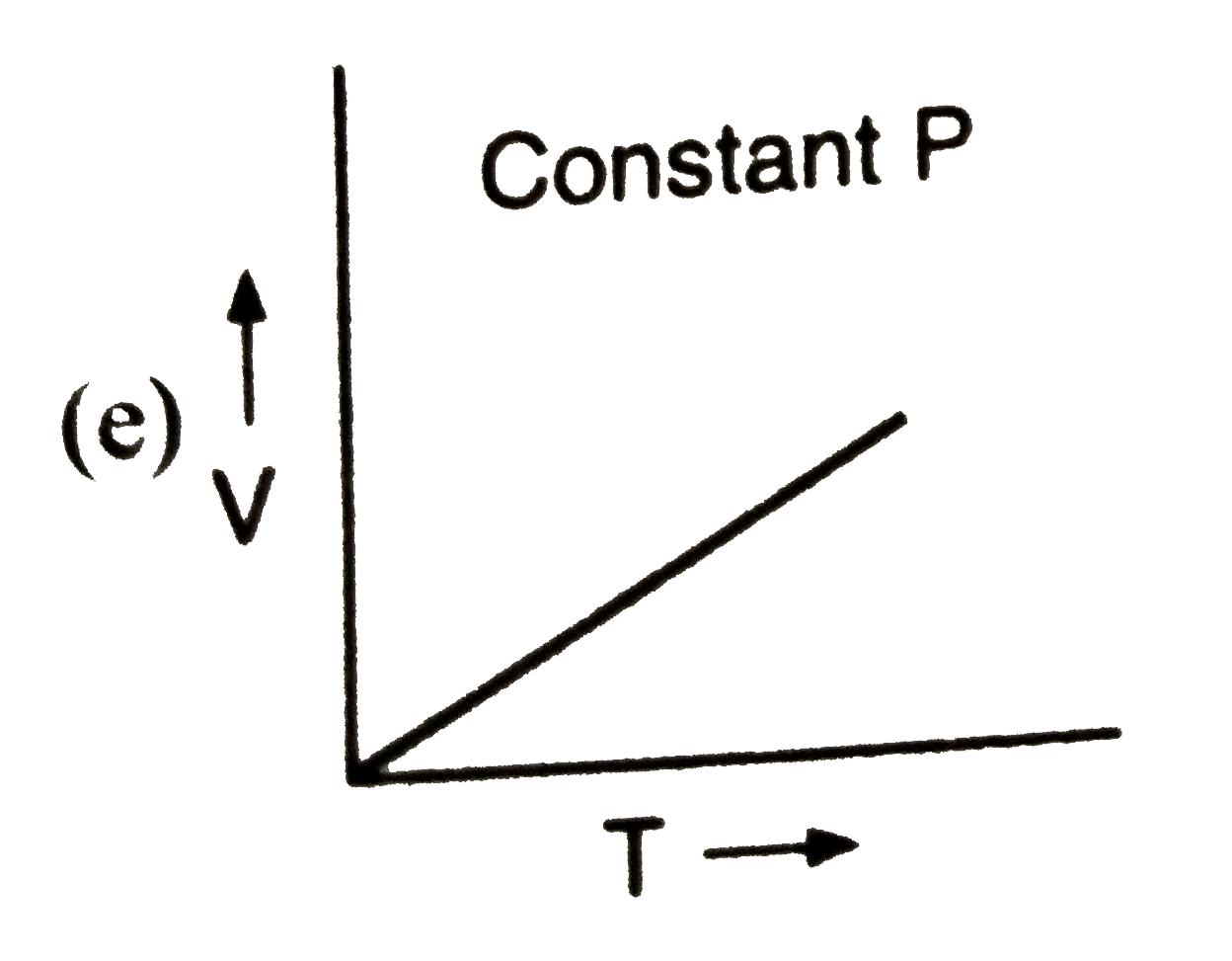
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions Level B|34 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Assertion-Reason|10 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Objective questions|105 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-Set 2
- The viscosity of a liquid molecule depends on :
Text Solution
|
- Viscosity si property of
Text Solution
|
- Which of the following diagrams correctly decribes the behavior of a f...
Text Solution
|
- The Ne atom has 10 times the mass of H(2) molecule. Which of the follo...
Text Solution
|
- Select the correct conditions indicated below the following plots :
Text Solution
|
- Assertion: Sulphur dioxide and chlorine are bleaching agents. Reason...
Text Solution
|
- Assertion: Nitrogen is unreactive at room temperature but becomes reac...
Text Solution
|
- Assertion: Noble gases can be liquefied. Reason: Attractive forces c...
Text Solution
|
- Assertion: Under similar conditions of temperature and pressure,O(2) d...
Text Solution
|
- Assertion: On compressing a gas to half the volume, the number of mole...
Text Solution
|
- Assertion: The plot of volume (V) versus pressure (P) at constant temp...
Text Solution
|
- Assertion: At constant temperature, if pressure on the gas is doubled,...
Text Solution
|
- Assertion: If H(2) and Cl enclosed separately in the same vessel exert...
Text Solution
|
- Assertion: Most probable velocity is the velocity possessed by maximum...
Text Solution
|
- Assertion: Compressibility factor (Z) for non ideal gases is always gr...
Text Solution
|
- Assertion: van der Waals equation is applicable only to non-ideal gase...
Text Solution
|
- Assertion: Helium shows only positive deviations from ideal behaviour....
Text Solution
|
- Assertion: Gases are easily absorbed on the surface of metals, especia...
Text Solution
|
- Assertion: SO(2) gas is easily liquefied while H(2) is not. Reason: ...
Text Solution
|
- (A) Diffusion is used in the enrichment of U^(235) (R) A lighter gas...
Text Solution
|