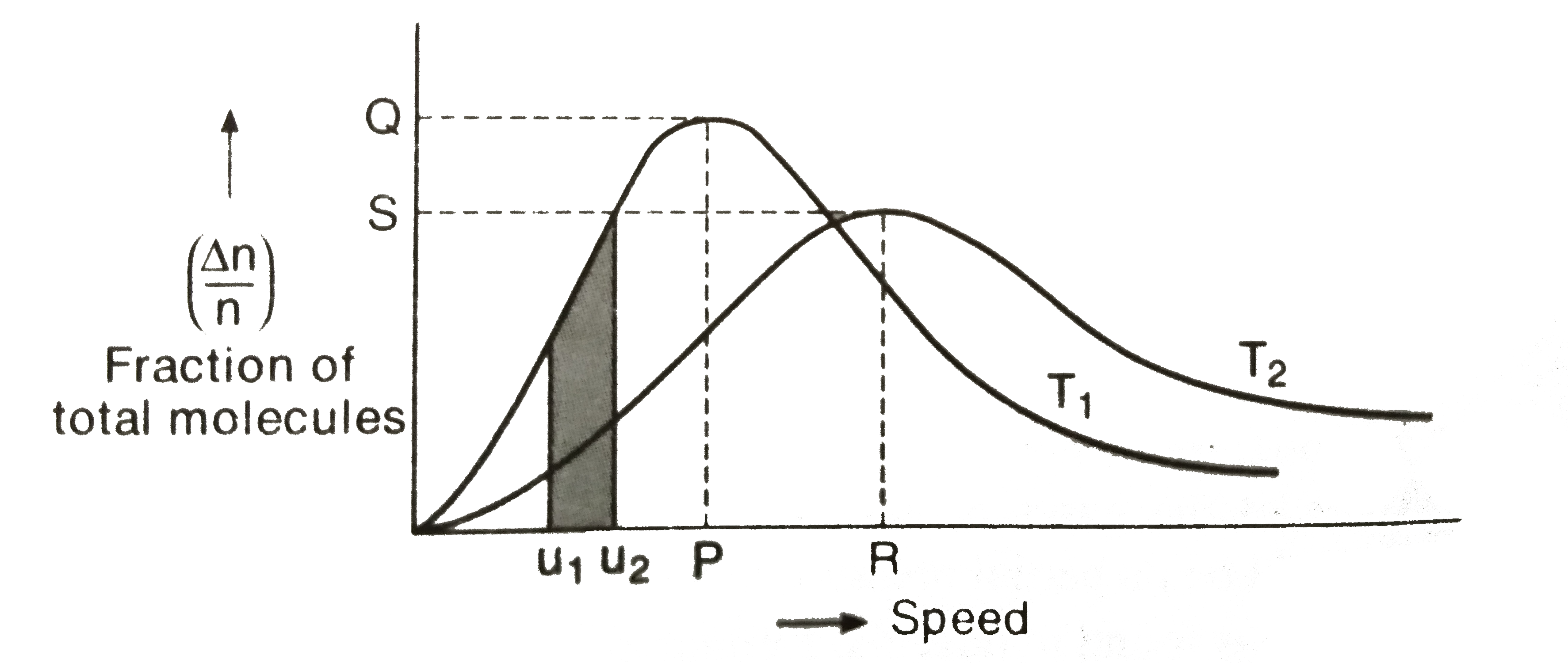
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise p 3|8 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise p 4|6 VideosSTATES OF MATTER (GASES AND LIQUIDS)
OP TANDON|Exercise Linked comprehension type question|6 VideosSOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)
OP TANDON|Exercise Self Assessment|31 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-STATES OF MATTER (GASES AND LIQUIDS)-p 2
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|
- The gas molecules randomly move in all directions and collide with eac...
Text Solution
|