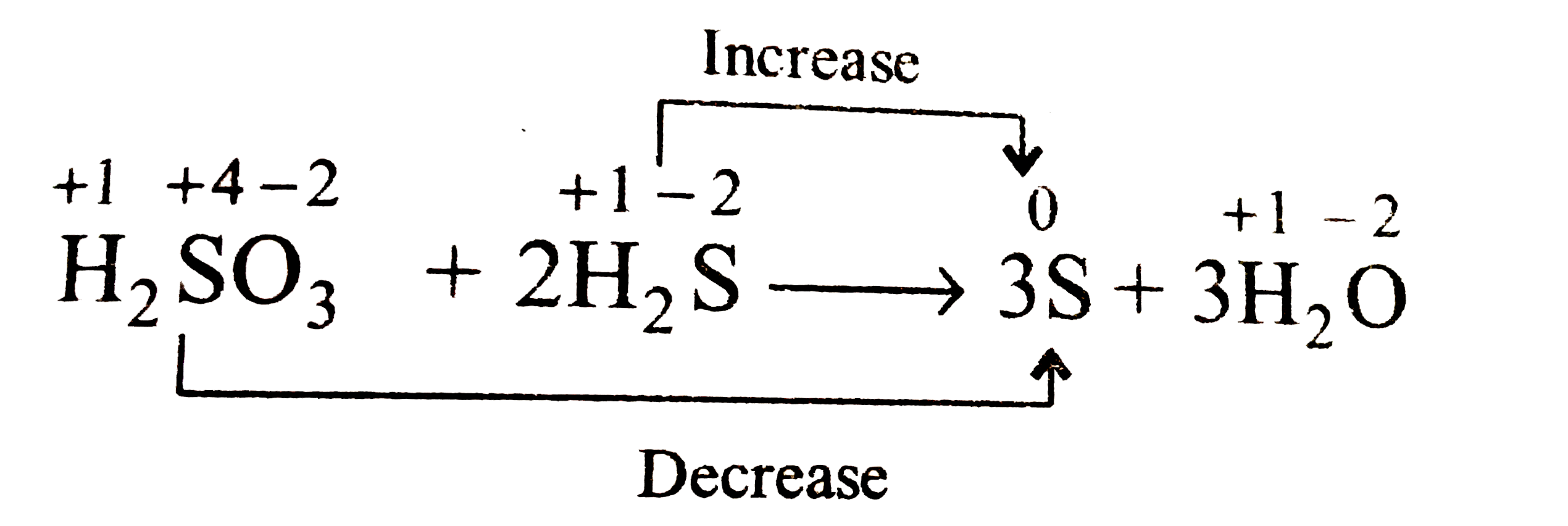
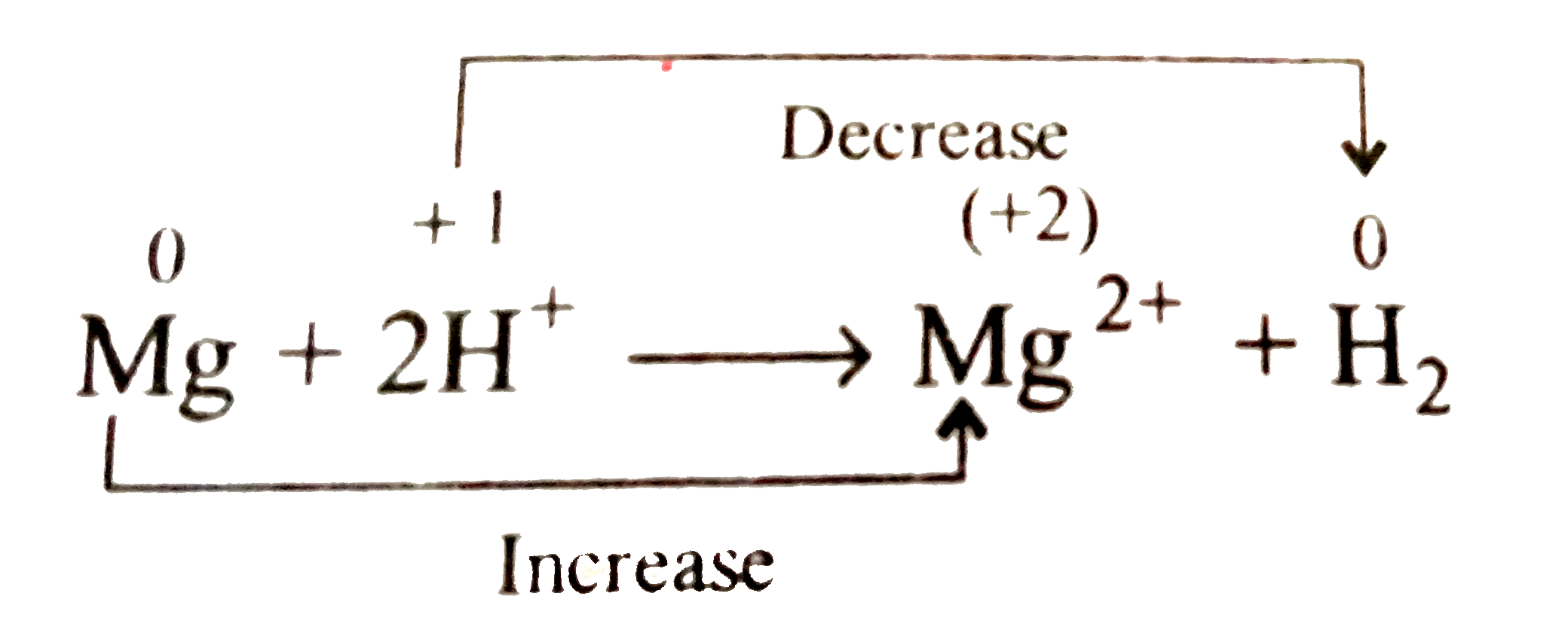Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the following reaction, identify the speech oxidised, the species r...
Text Solution
|
- In the following reaction, identify the speech oxidised, the species r...
Text Solution
|
- Which substance is oxidised and which substance/ion is reduced in the ...
Text Solution
|
- निम्नलिखित अभिक्रियाओं में ऑक्सीकारक तथा अपचायक बताइए - (i) 2FeCl(3)...
Text Solution
|
- Name the substance oxidised and the substance reduced, and also identi...
Text Solution
|
- Identify oxidising are reducing agents in the following reactions : ...
Text Solution
|
- निम्न अभिक्रियाओं में ऑक्सीकारक व अपचायक छाँटिए - (i) 2FeCl(3...
Text Solution
|
- निम्नलिखित अभिक्रियाओं में से ऑक्सीकारक व अपचायक बताइए - 2FeCl(3)+Sn...
Text Solution
|
- Name the oxidising and reducing agent in the following reaction: 2H(...
Text Solution
|