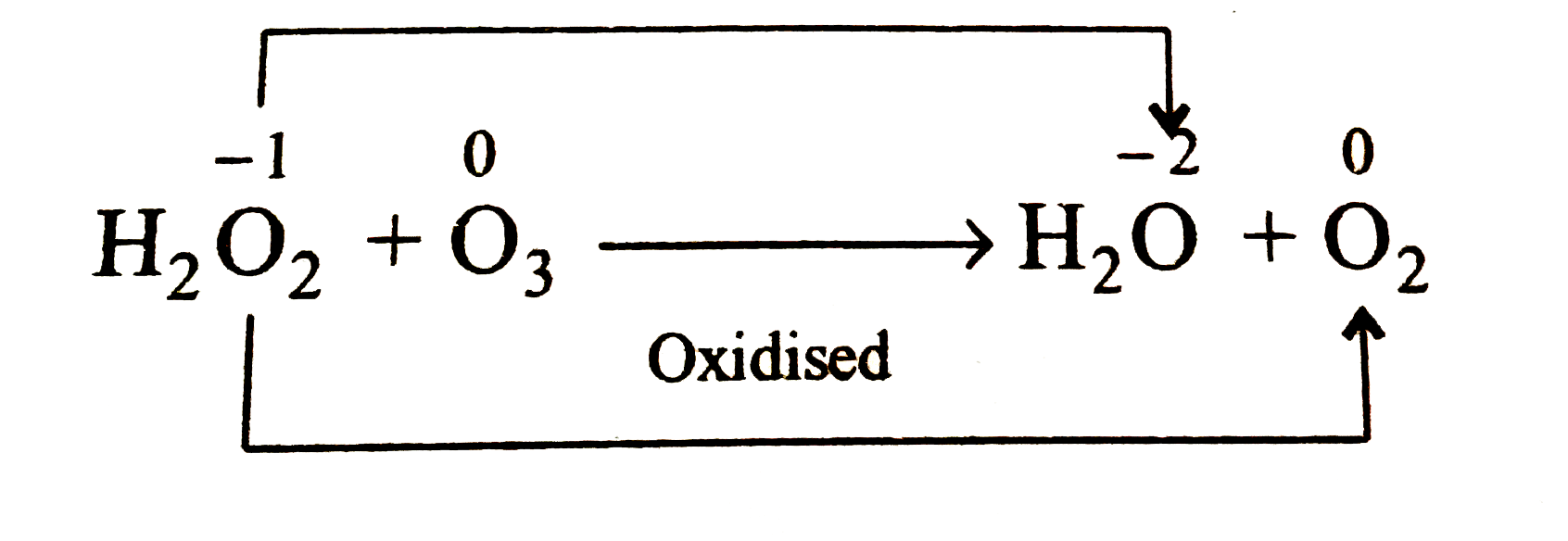Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Balance the following chemical equation H(2)O(2)+O(3) to H(2)O+O(2) ...
Text Solution
|
- In the reaction Ag(2)O+H(2)O(2) rarr 2Ag+H(2)O+O(2) , H(2)O(2) acts as
Text Solution
|
- The oxidation number of oxygen in H(2)O(2)is
Text Solution
|
- Role of hydrogen peroxide iin the following reaction is respectively. ...
Text Solution
|
- Balance the following equation by oxidation number method PbS+H(2)O(2)...
Text Solution
|
- Balance the following chemical equation H(2)O(2)+O(3) to H(2)O+O(2) In...
Text Solution
|
- In the reaction , Ag(2)O+ H(2)O(2) to 2Ag + H(2)O+ O(2) H(2)O(2) acts ...
Text Solution
|
- निम्नलिखित अभिक्रिया में ऑक्सीकारक छाँटिए- H(2)O(2)+O(3) to H(2)O+ 2...
Text Solution
|
- O(3) तथा H(2)O(2) में ऑक्सीजन की ऑक्सीकरण संख्याएँ है क्रमशः
Text Solution
|