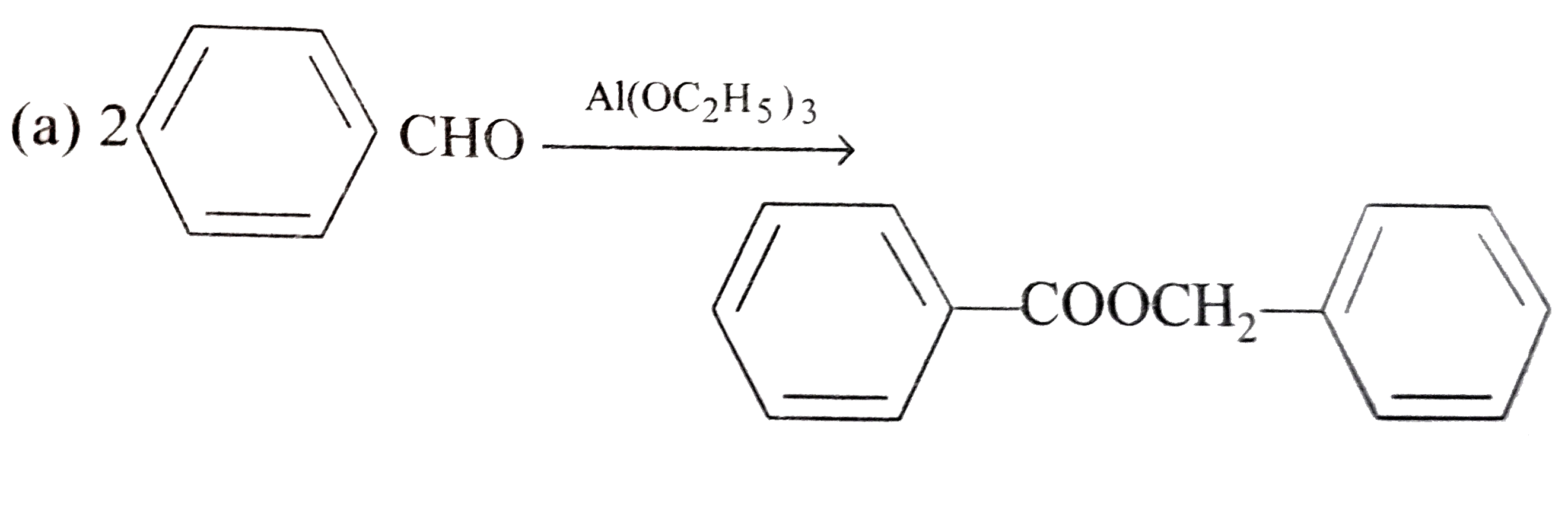
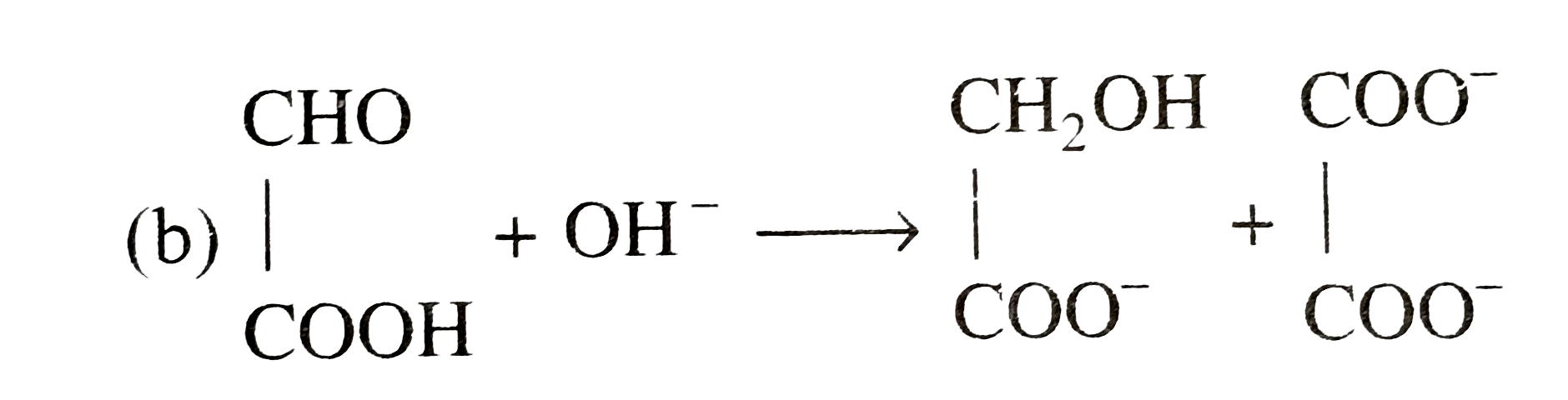A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
OXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise SECTION B|35 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise STEP II|7 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise Short answer|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 VideosPOLYMERS
OP TANDON|Exercise PASSAGE 3|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-OXIDATION AND REDUCTION (REDOX REACTIONS)-LEVEL A
- The reaction 3ClO^(-)(aq)rarrClO(3)^(-)(aq)+2Cl^(-)(aq) an example of ...
Text Solution
|
- The oxidation state of 'S' in H(2)SO(4) is:
Text Solution
|
- Which of the following is not a disproportionation reaction ?
Text Solution
|
- Which of the following is a disproportionation reaction?
Text Solution
|
- When KMnO(4) acts as an oxidising agnet and ultimetely from MnO(4)^(2-...
Text Solution
|
- which of the following is a redox reaction ?
Text Solution
|
- For decolourisation of 1 "mol of" KMnO(4), the moles of H(2)O(2) requi...
Text Solution
|
- The reaction of KMnO(4) and HCI results in:
Text Solution
|
- Consider the following reaction, 5H(2)O(2)+ClO(2)+2OH^(-) to Cl^(-)+...
Text Solution
|
- In the chemical reaction, Ag(2)O+H(2)O+2e^(-) to 2Ag+2OH^(-)
Text Solution
|
- The reaction, 2H(2)O(l) to 4H^(+)(aq.)+O(2)(g)+4^(-) is
Text Solution
|
- Which of the following molecules can act as an oxidating as well as a ...
Text Solution
|
- Which of the following is not a reducing agent ?
Text Solution
|
- Equivalent mass of oxidizing agent in the reaction, SO(2)+2H(2)S rar...
Text Solution
|
- Among these, identify the species with an atom in +6 oxidation state: ...
Text Solution
|
- On reduction of KMnO(4) by oxalic acid in acidic medium, the oxidation...
Text Solution
|
- The number of moles of K(2)Cr(2)O(7) reduced by 1 mol of Sn^(2+) ions ...
Text Solution
|
- In the standardization of Na(2)S(2)O(3) using K(2)Cr(2)O(7) by iodomet...
Text Solution
|
- In the balanced chemical reaction IO(3)^(ө)+al^(ө)+bH^(ө)rarrcH(2)O+...
Text Solution
|
- In alkaline medium CIO(2) oxidises to H(2)O(2) and O(2) and itself get...
Text Solution
|