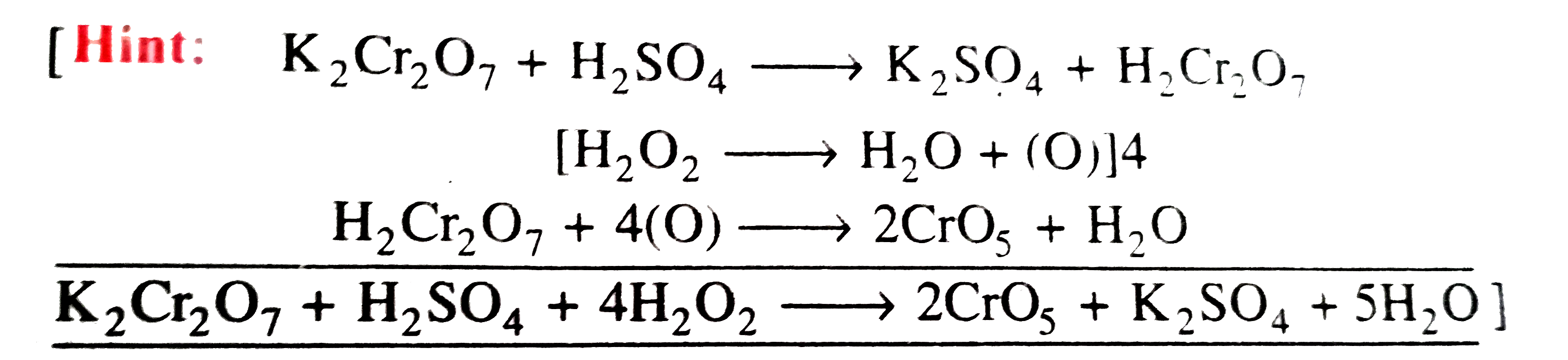A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
OXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise SECTION B|35 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise STEP II|7 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise Short answer|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 VideosPOLYMERS
OP TANDON|Exercise PASSAGE 3|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-OXIDATION AND REDUCTION (REDOX REACTIONS)-LEVEL A
- The oxidation numbers of the sulphur atoms in pcroxy- monosulphuric ac...
Text Solution
|
- When phosphorus reacts with caustic soda, the products are PH(3) and N...
Text Solution
|
- When hydrogen peroxide is added to acidified potassium dichromate, a b...
Text Solution
|
- Number of moles of MnO(4)^(-) required to oxidise one mole of ferrous ...
Text Solution
|
- Oxidation number if iodine in IO(3)^(-), IO(4)^(-),KI and I(2) respect...
Text Solution
|
- In the redox reaction, x KMnO(4) + NH(3) rarr y KNO(3) + MnO(2) + MnO(...
Text Solution
|
- The reaction 3ClO^(-)(aq)rarrClO(3)^(-)(aq)+2Cl^(-)(aq) an example of ...
Text Solution
|
- Which of the following species does not show disproportionation reacti...
Text Solution
|
- Which of the following shows nitrogen with its increasing order of oxi...
Text Solution
|
- Freshly prepared, bright blue coloured solution of sodium in liquid am...
Text Solution
|
- The reaction of white phosphorus with aqueous NaOH gives phosphine alo...
Text Solution
|
- In the oxidation of sulphite to sulphate using pennanganate the number...
Text Solution
|
- Which ordering of Compounds is according to the decreasing order of th...
Text Solution
|
- Consider the following reaction : xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+)...
Text Solution
|
- In which of the following reactions H(2)O(2) acts as reducing agent? ...
Text Solution
|
- In which of the following coordination compounds, the central metal ir...
Text Solution
|
- The pair in which phosphours atoms have a formed oxidation state of +3...
Text Solution
|
- Which of the following reactions is an example of a redox reaction? ...
Text Solution
|
- The oxidation states of Cr in [Cr(H(2)O)(6)]Cl(3). ,[Cr(C(6)H(6))(2)...
Text Solution
|
- In this reaction ozone acts as:
Text Solution
|