A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
OXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise STEP II|7 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise Assertion Reason|14 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise LEVEL A|131 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 VideosPOLYMERS
OP TANDON|Exercise PASSAGE 3|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-OXIDATION AND REDUCTION (REDOX REACTIONS)-SECTION B
- The number of moles of K(2)Cr(2)O(7) reduced by 1 mol of Sn^(2+) ions ...
Text Solution
|
- The3ClO^(-)(aq.)to ClO(3)^(-)(aq.)+2Cl^(-)(aq.) is an example of
Text Solution
|
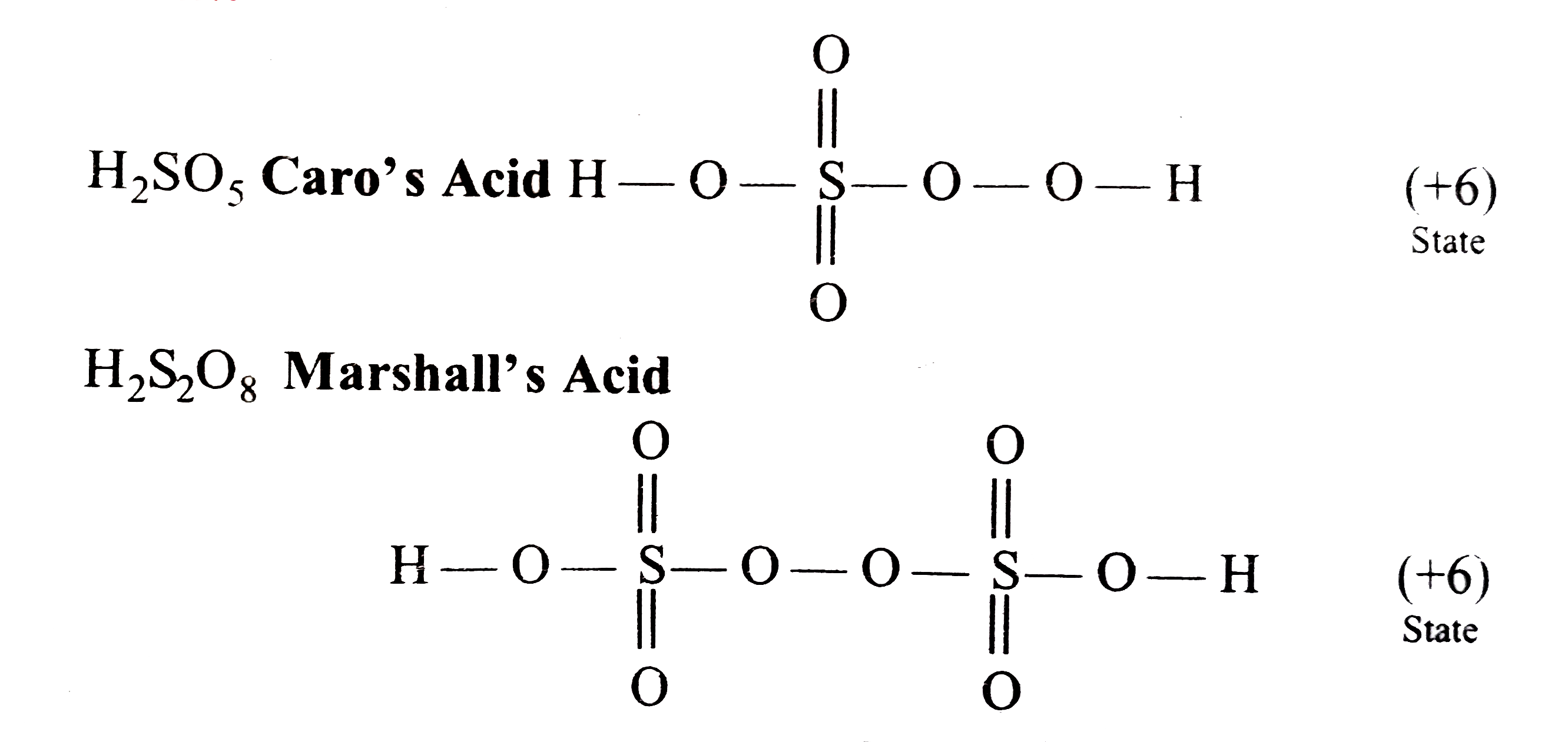
- The oxidation state of S-atoms in Caro's and Marshall's acids are:
Text Solution
|
- Which among the following compounds have +6 state with the metal atoms...
Text Solution
|
- The oxidation number of nitrogen atom in NH(4)NO(3) are:
Text Solution
|
- In the chemical reaction, K(2)Cr(2)O(7)+xH(2)SO(4)+ySO(2)rarrK(2)SO(...
Text Solution
|
- In which of the following pairs both members contain peroxy linkage?
Text Solution
|
- Which of the following agents is the most oxidising?
Text Solution
|
- When methane is burnt in oxygen to produce CO(2) and H(2)O. the oxidat...
Text Solution
|
- Which of the following has been arranged m order of increasing oxidati...
Text Solution
|
- In the ethylene molecule the two carbon atoms have the oxidation numbe...
Text Solution
|
- In which of the following coordmation compounds do the transition meta...
Text Solution
|
- In the redox reaction xMnO+yPbO(2)+zHNO(3) rarr HMnO(4)+Pb(NO(3))(2)...
Text Solution
|
- In the redox reaction, x KMnO(4) + NH(3) rarr y KNO(3) + MnO(2) + MnO(...
Text Solution
|
- In a redox reaction: xCH(3)CH(2)OH+yI(2)+zOH^(-) to CHO(3)+HCO(2)^(-...
Text Solution
|
- The oxidation number of carboxylic carbon atom in CH(3)COOH is
Text Solution
|
- Oxidation state of nitrogen is incorrectly given for:
Text Solution
|
- Oxidation number of C in HNC is:
Text Solution
|
- Which of the following groups of molecules act both as oxidising agent...
Text Solution
|
- The oxidising state of molybdenum in its oxo complex species [Mo(2)O...
Text Solution
|