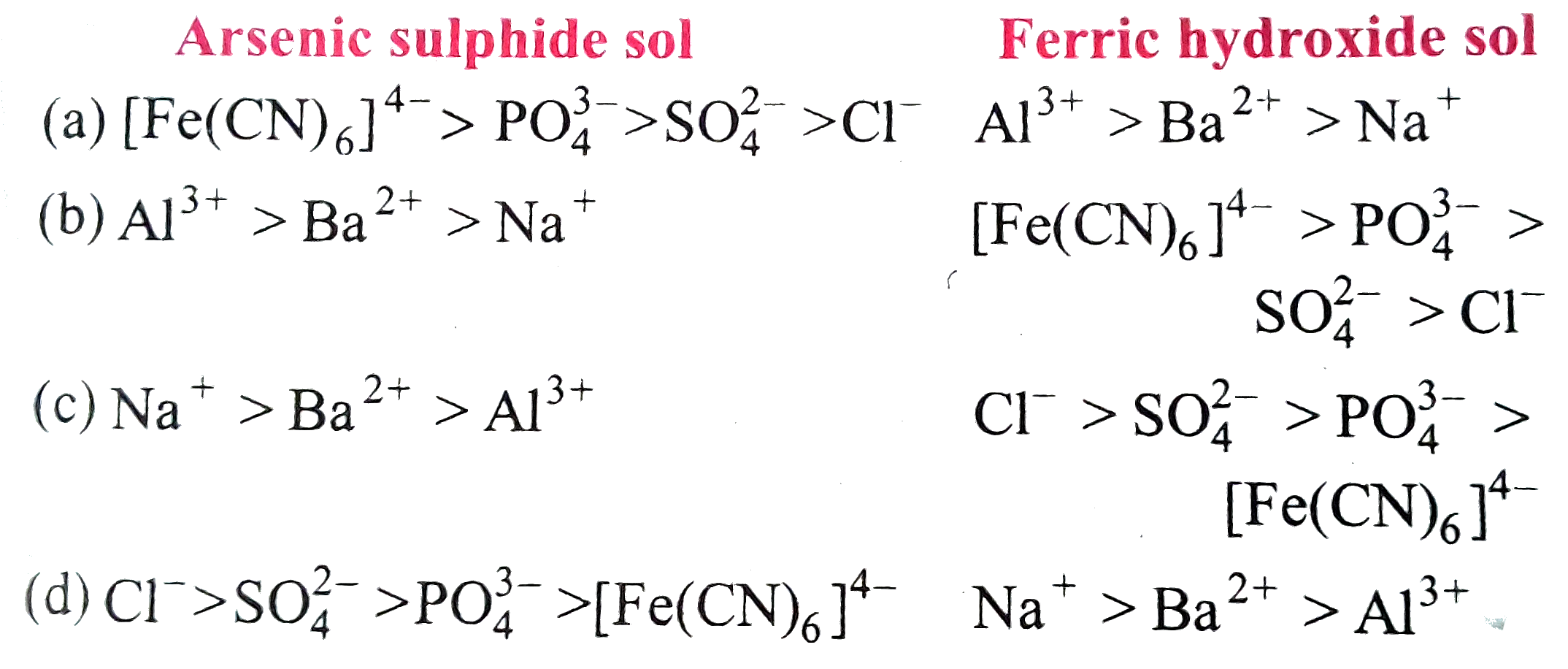
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE COLLOIDAL STATE
OP TANDON|Exercise Step -2 Objective|10 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise assertion- Reason|15 VideosTHE COLLOIDAL STATE
OP TANDON|Exercise Objective Questions|140 VideosSTATES OF MATTER (SOLID STATE)
OP TANDON|Exercise SELF ASSESSMENT Section VI|3 VideosVOLUMETRIC ANALYSIS
OP TANDON|Exercise Section-V|3 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-THE COLLOIDAL STATE-step-1 Objective
- Which of the following statements are correct ?
Text Solution
|
Text Solution
|
- The flocculating power of the given ions for the specified colloidal s...
Text Solution
|
- The coagulation value in millimoles per litre of electolytes used for ...
Text Solution
|
- Cotrell precipitator works on the principle of :
Text Solution
|
- The substances involved in micellization are :
Text Solution
|
- The swelling of 'gel' when placed in water is called:
Text Solution
|
- A lyophilic sol is at its isoelectric point then it is :
Text Solution
|
- Sedimentation potential is reverse of :
Text Solution
|
- The potential differnce between the fixed particles layer and the diff...
Text Solution
|
- Silver iodide is used for producing artificial rains because Agl:
Text Solution
|
- All colloidal solutions show :
Text Solution
|
- Colloidation is a colloidal solution of :
Text Solution
|
- During micelle formation :
Text Solution
|
- Which of the following is not the property of hydrophilic solutions ?
Text Solution
|
- The coagulation of 100ml of colloidal solution of gold is completely p...
Text Solution
|
- Select the non- eleastic gel out of the following :
Text Solution
|
- The colligative property of a colloidal sol compared to the solution o...
Text Solution
|
- 1 mole of Agl//Ag^+ sol. Is coagulated by :
Text Solution
|
Text Solution
|