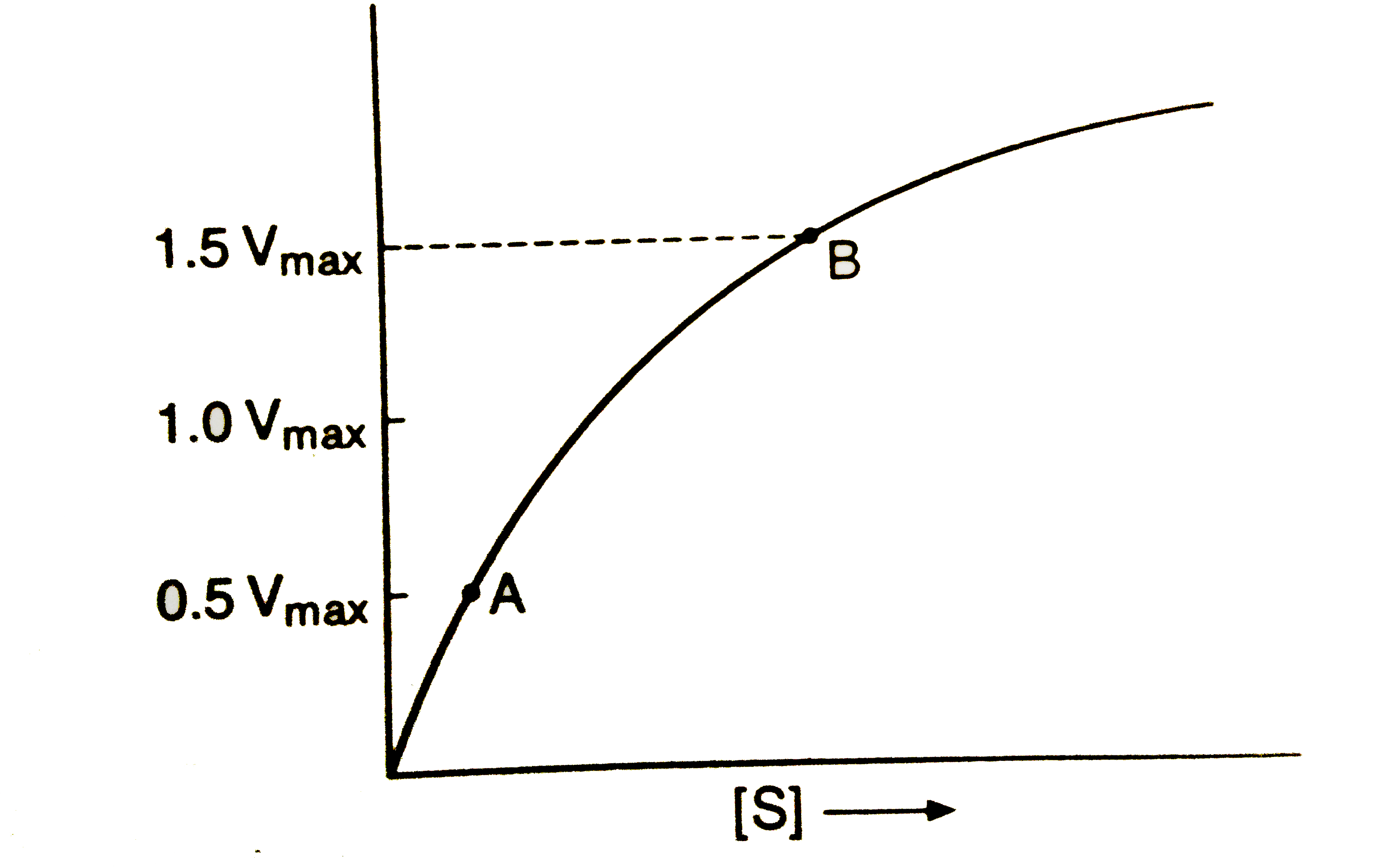
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ADSORPTION AND CATALYSIS
OP TANDON|Exercise Assertion -Reason TYPE QUESTIONS|19 VideosADSORPTION AND CATALYSIS
OP TANDON|Exercise Matrix Matching Type Questions|7 VideosADSORPTION AND CATALYSIS
OP TANDON|Exercise OBJECTIVE QUESTIONS (LEVEL-A)|119 VideosAROMATIC HYDROCARBONS (ARENES)
OP TANDON|Exercise EXAMPLES|24 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ADSORPTION AND CATALYSIS-OBJECTIVE QUESTIONS (LEVEL-B)
- The curveshowing the variation of pressure with temperature for a give...
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Adsorption is the tendency of accumulation of molecular species at the...
Text Solution
|
- Adsorption is the tendency of accumulation of molecular species at the...
Text Solution
|
- Which of the following factors are responsible for the increase in the...
Text Solution
|
- In Langumir's model of adosrption of a gas on a solid surface :
Text Solution
|
- An enzyme [E] is combined with the substrate [S] as follows: E+S und...
Text Solution
|
- Which type of graph gives straight line in Langmuir adsorption isother...
Text Solution
|
- Plot of log against log P is a straight line inclined at an angl...
Text Solution
|
- Following graphs will be true when :
Text Solution
|
- Methylene blue, from its aqueous solution, is adsorbed on activated ch...
Text Solution
|
- Zeolites are microporous catalyst. General formula of Zeolite may be g...
Text Solution
|
- Which of the following statements are correct ?
Text Solution
|
- Which of the following are Zeolites ?
Text Solution
|
- Which of the following act as negative catalyst ?
Text Solution
|
- Select the correct statements about enzymes :
Text Solution
|
- Which of the following are correct about the catalyst ?
Text Solution
|
- The given graph/data I,II,III and IV represent general trends observe...
Text Solution
|
- When O2 is adsorbed on ametallic surface, electron transfer occurs fro...
Text Solution
|
- The correct statement (s) about surface properties is (are)
Text Solution
|