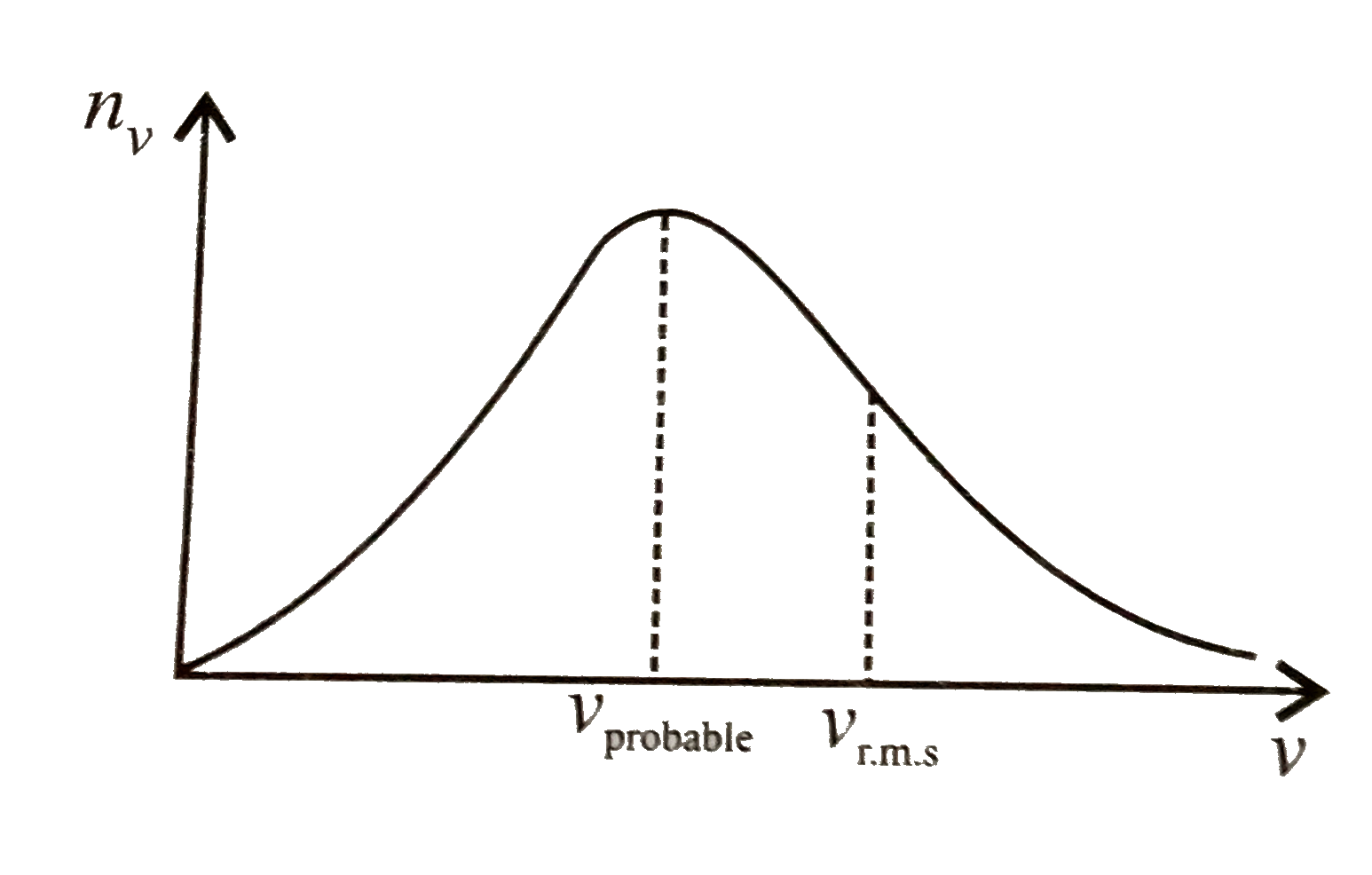
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-KINETIC THEORY-Assertion And Reason
- Assertion: All molecules of an ideal gas more with the same speed. R...
Text Solution
|
- Assertion: In a mixture of gases at a fixed temperatue, the heavier mo...
Text Solution
|
- Assertion: In case of collision of gas molecules in a given amount of ...
Text Solution
|
- Assertion : The rms velocity and most probable speeds of the molecules...
Text Solution
|
- Assertion: The ratio of rms speed and average speed of a gas molecules...
Text Solution
|
- Assertion: Average kinetic energy per molecule of any ideal monoatomic...
Text Solution
|
- Assertion: For a mixture of non reactive ideal gases, the total pressu...
Text Solution
|
- Assertion: Each vibrational mode gives two degrees of freedom. Reaso...
Text Solution
|
- Assetion : Specific heat of a gas at constant pressure is greater than...
Text Solution
|
- Assertion : The ratio C(P)// C(upsilon) for a diatomic gas is more tha...
Text Solution
|