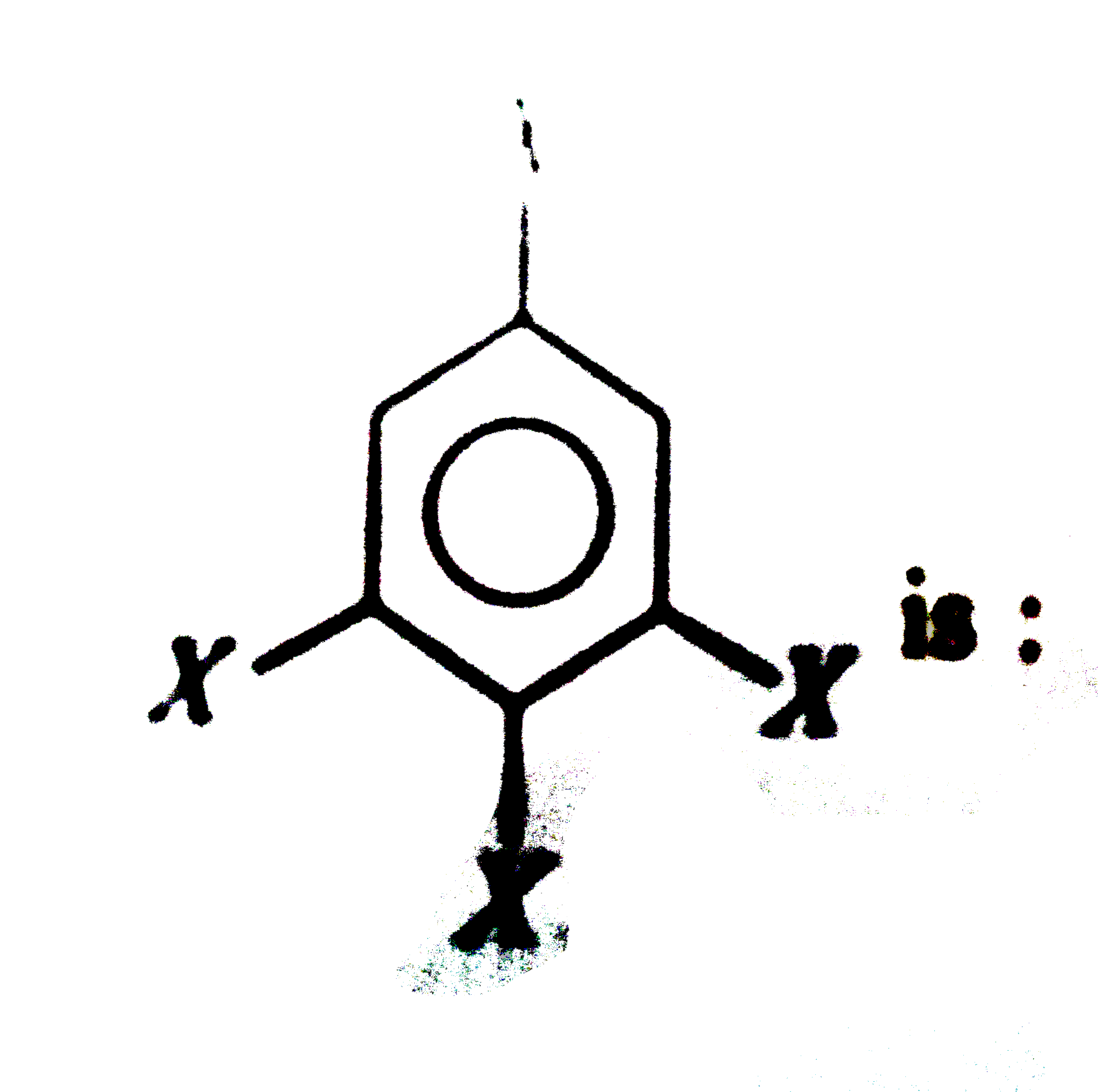A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise OBJECTIVE QUESTIONS (Level-B)|46 VideosINTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise ASSERTION-REASON TYPE|14 VideosINTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise PROBLEMS FOR PRACTICE|12 VideosCHEMISTRY IN EVERYDAY LIFE
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-INTRODUCTION TO ORGANIC CHEMISTRY -OBJECTIVE QUESTIONS (Level-A)
- The CI - C - CI angle in 1, 1, 2, 2, tetrachloroethone and tetrachloro...
Text Solution
|
- Which of the following molecules does not have net dipole moment?
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Arrange the following molecules in the correct order of decreasing C-C...
Text Solution
|
- The bond energy (in kcal mol^(-1)) of a C -c single bond is approximat...
Text Solution
|
- Which of the following pairs have identical bond order?
Text Solution
|
- The correct order of bond energy is:
Text Solution
|
- All carbon atoms are sp^(2)-hybridised in:
Text Solution
|
- Resonance occurs due to the :
Text Solution
|
- Resonance structures of a molecule do not have:
Text Solution
|
- Maximum number of sigma bonds that may be present in an isomer of C(4)...
Text Solution
|
- How many sigma and pi-bonds are there in the molecule of dicyanoethene...
Text Solution
|
- Which of the following compounds shows evidence of the strongest hydro...
Text Solution
|
- The hydrogen bond is strongest in which one of the following?
Text Solution
|
- Increasing order of carbon-carbon bond length for the following is: ...
Text Solution
|
- The correct order of boiling points of hydrogen halide is:
Text Solution
|
- Among the following, the correct order of acidity is:
Text Solution
|
- The state of hybridization of C(2),C(3),C(5) and C(6) of the hydrocarb...
Text Solution
|
- Types of hybridisation of carbon atoms noted as 1 and 2 are: H(2)C=o...
Text Solution
|
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of