A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise OBJECTIVE QUESTIONS (Level-B)|46 VideosINTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise ASSERTION-REASON TYPE|14 VideosINTRODUCTION TO ORGANIC CHEMISTRY
OP TANDON|Exercise PROBLEMS FOR PRACTICE|12 VideosCHEMISTRY IN EVERYDAY LIFE
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-INTRODUCTION TO ORGANIC CHEMISTRY -OBJECTIVE QUESTIONS (Level-A)
- Consider the following compounds: (A) chloroethene (B) benzene (C) ...
Text Solution
|
- The decreasing order of blood dissociation energies of C-C, C-H and H-...
Text Solution
|
- In 2-butene, which one of the following statements is true?
Text Solution
|
- Compare List I and List II and choose the correct matching codes from ...
Text Solution
|
- In the following compound, the number of 'sp' hybridised carbon is: ...
Text Solution
|
- Which of the following has the highest dipole moment?
Text Solution
|
- Among the following, the least stable resonance structure is :
Text Solution
|
- Which one of the following arrangements does not give the correct pict...
Text Solution
|
- The enolic form of ethyl acetoacetate as shown below has
Text Solution
|
- The enolic form of butanone contains
Text Solution
|
- Hybridization of nitrogen atom in pyridine is:
Text Solution
|
- The number of pi-bonds in the following compound O(2)N-C-=C-NO(2) is:
Text Solution
|
- The compound in which underlined carbon uses only its sp^(3)-hybrid or...
Text Solution
|
- Match the following: {:((A)"Simple distillation",(i)"to separate the...
Text Solution
|
- Match the following: {:((A)"Sublimation",(i)"Ether+toluene"),((B)"Di...
Text Solution
|
- Allyl cyanide has
Text Solution
|
- The correct order of decreasing H-C-H angle in the following molecule ...
Text Solution
|
- For which of the following molecule significant mu ne0 ?
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- The distillation technique most sited for separating glycerol from spe...
Text Solution
|
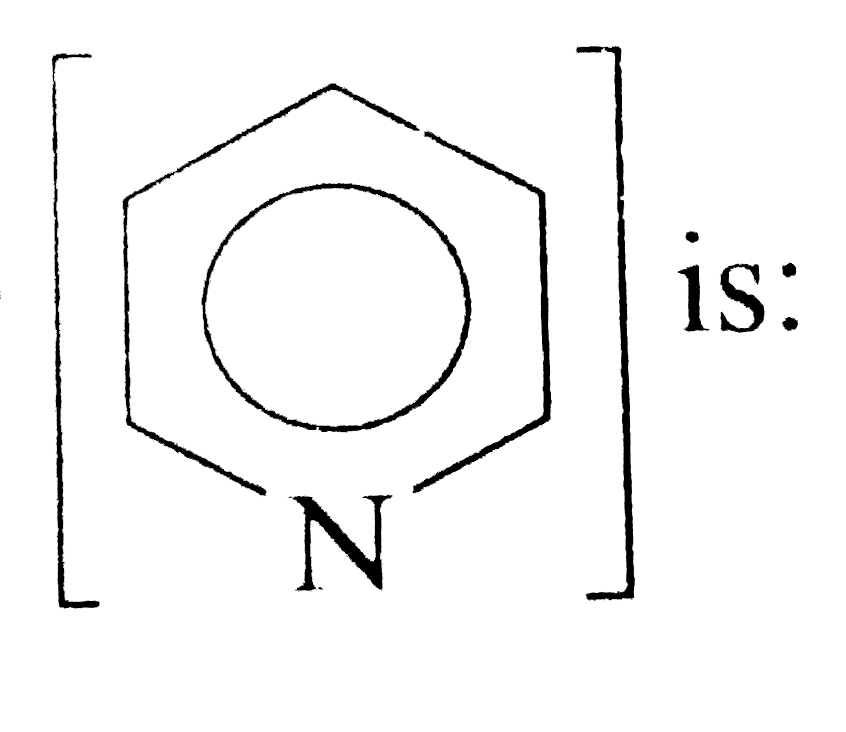 is:
is: