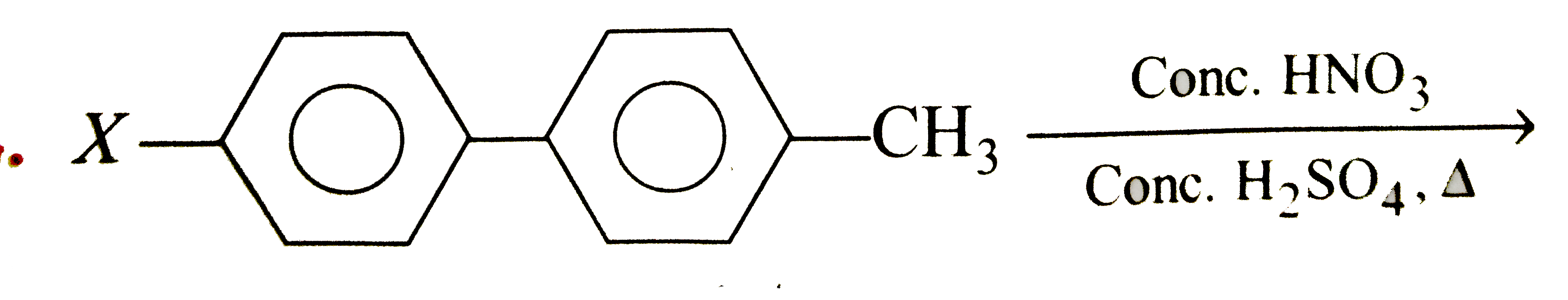
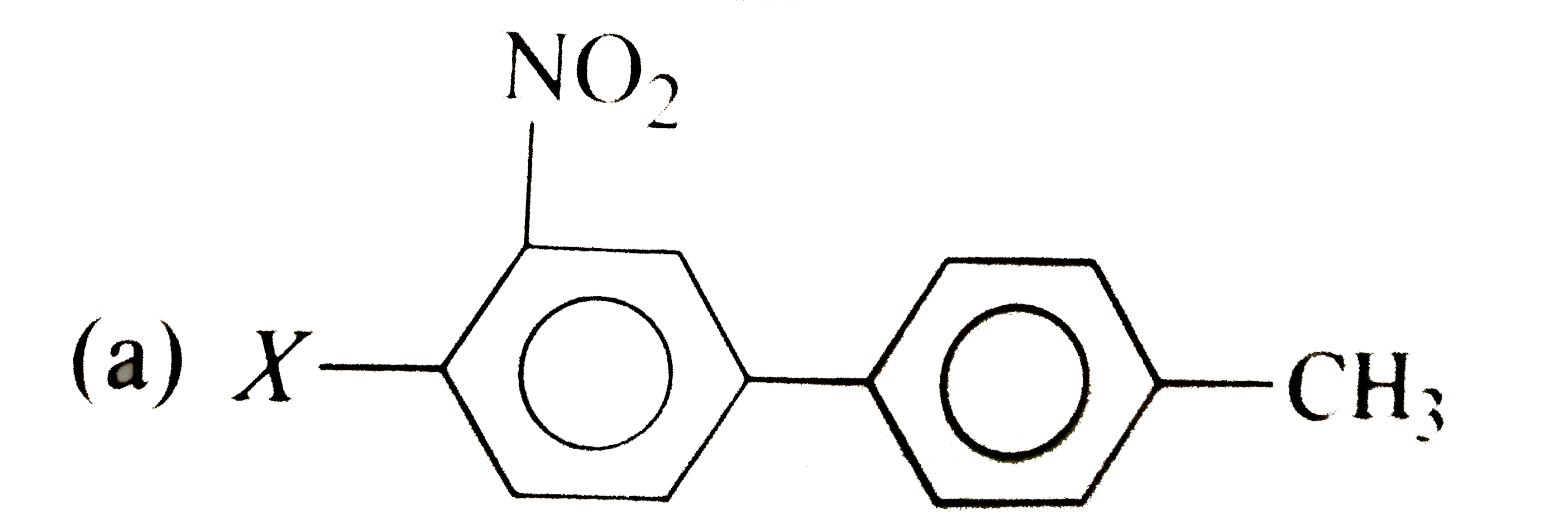
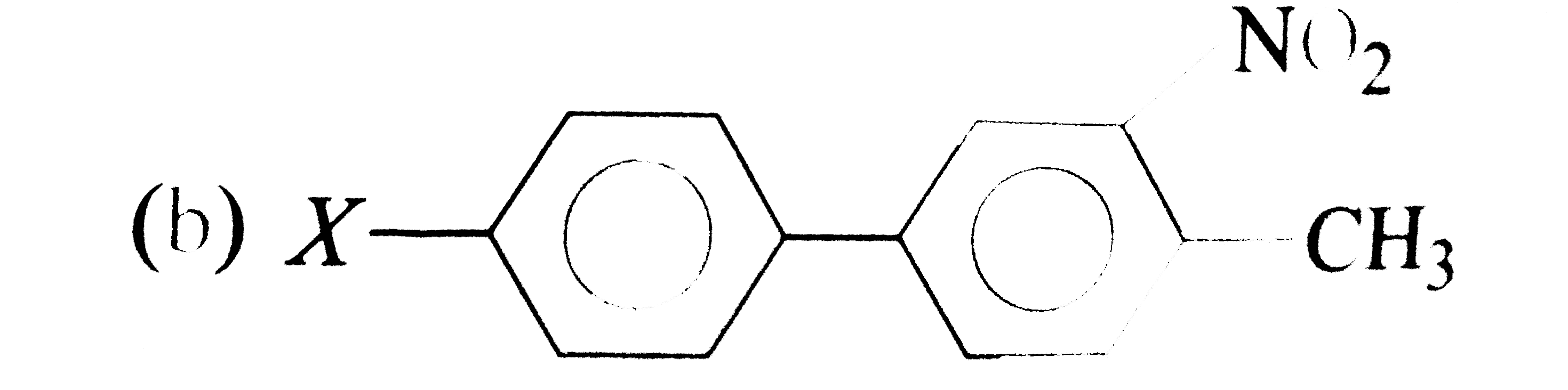
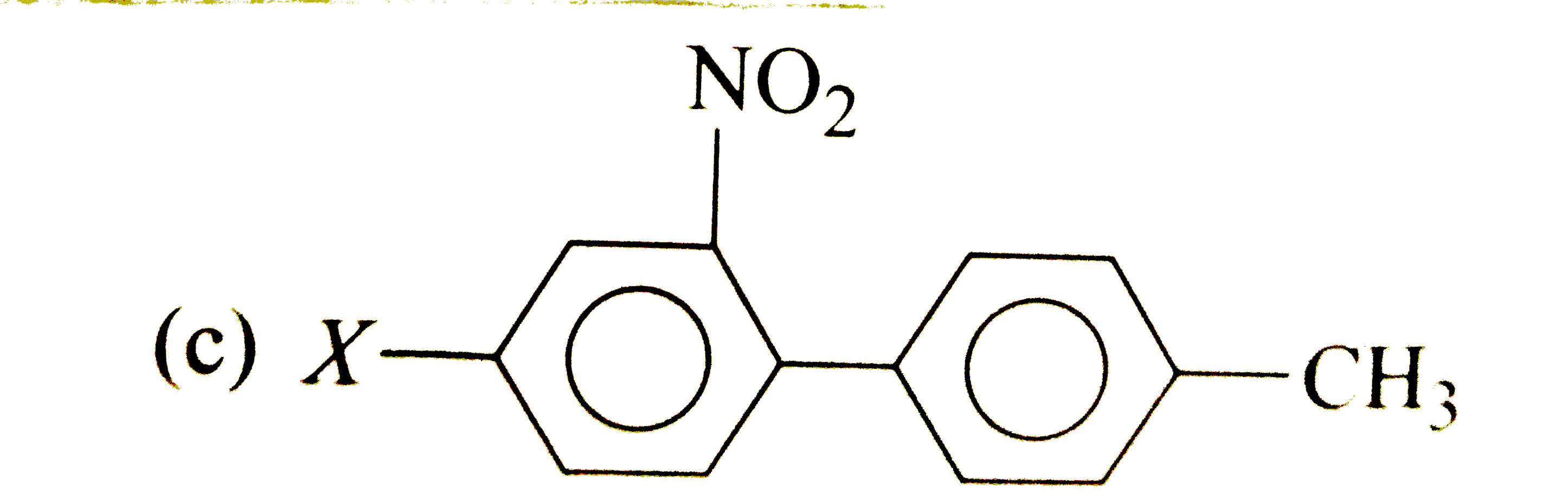
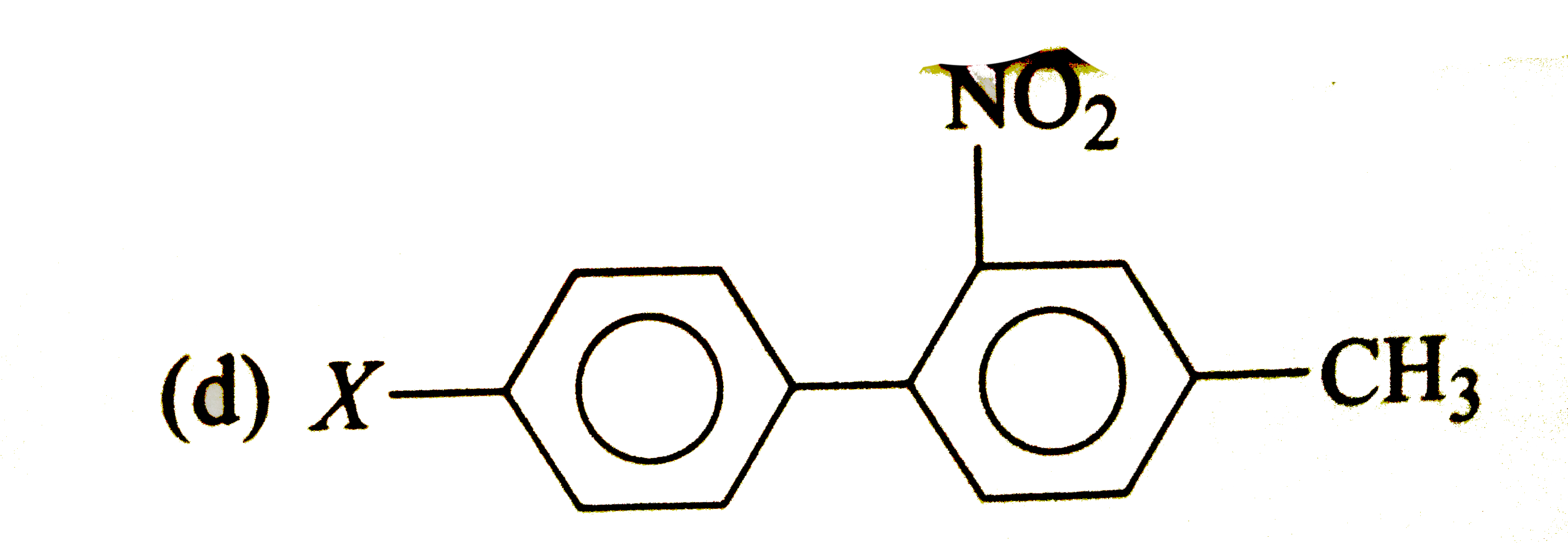
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|
- If aromatic ring is substituted by more than one group then electrophi...
Text Solution
|
- If aromatic ring is substituted by more than one group then electrophi...
Text Solution
|
- If aromatic ring is substitued by more than groups then electrophilic...
Text Solution
|
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|
- Aromatic hydrocarbon can show electrophilic substitution reaction, oxi...
Text Solution
|