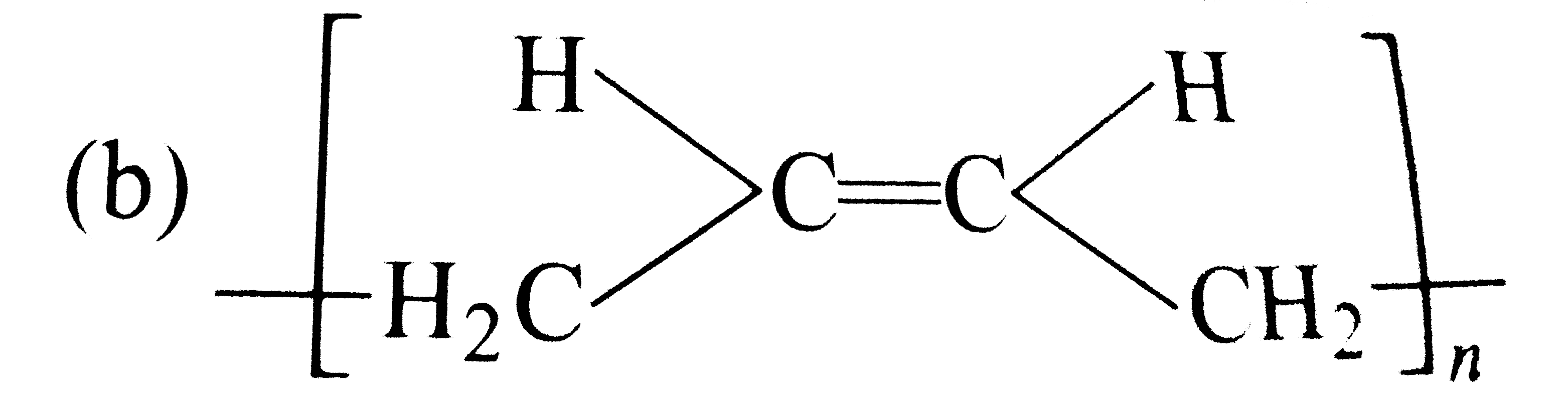
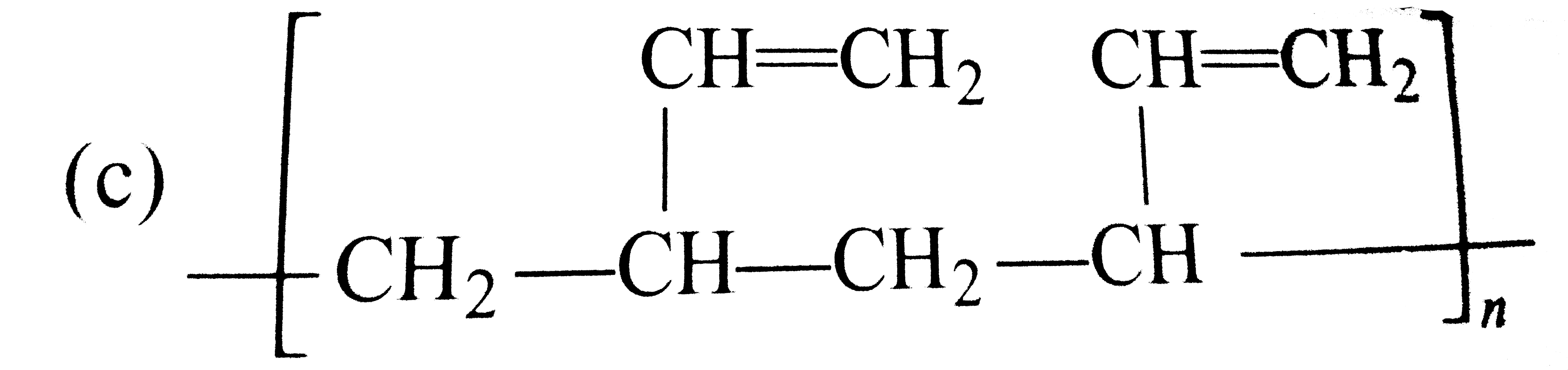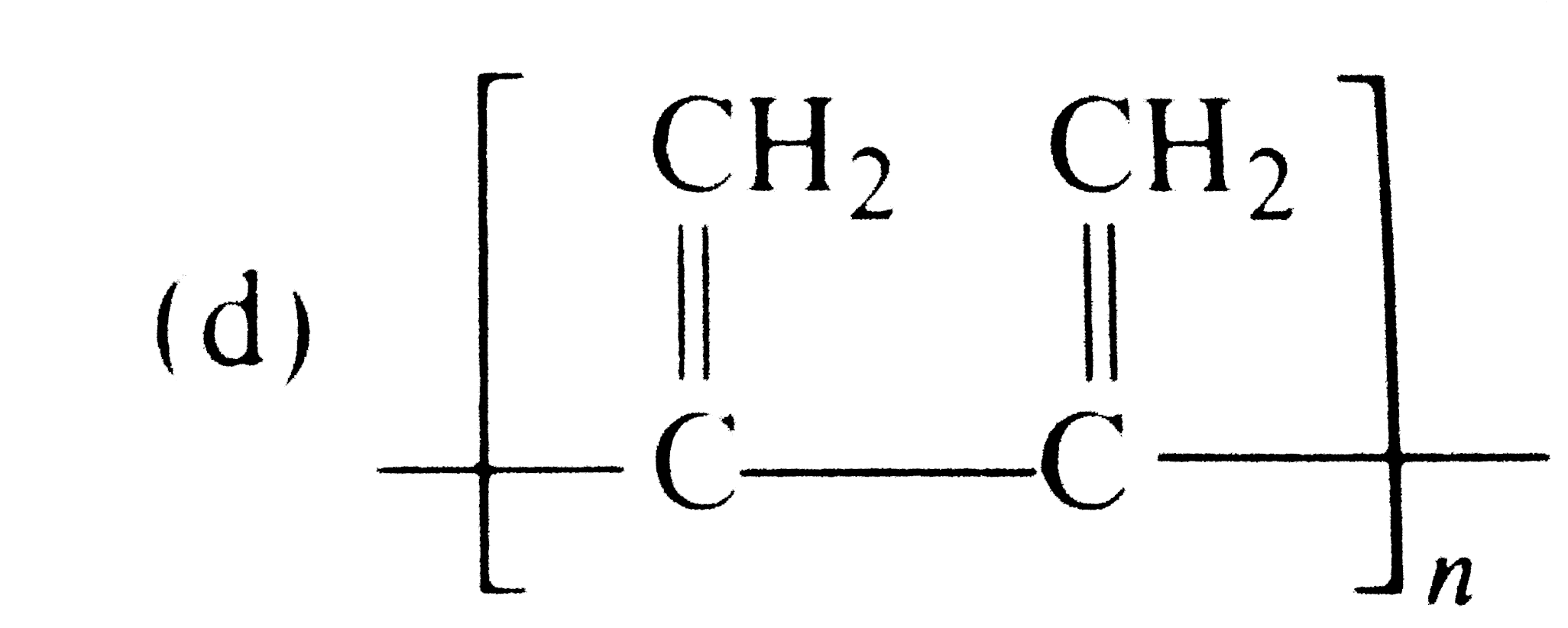A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Mark out the most unlike form of polymerization of H(2)C=CH-CH=CH(2)
Text Solution
|
- Name of the following as substituted derivatives of ethylene. (a) C...
Text Solution
|
- What hybrid orbitals will form the following compound H(3)C-CH=CH-CH(2...
Text Solution
|
- The IUPAC name of CH(2) =CH-CH=CH-underset(CH=CH(2))underset(|)(CH)-C-...
Text Solution
|
- Addition of HBr on, CH-=C-CH(2)-CH=CH(2) and CH-=C-CH=CH(2) separately...
Text Solution
|
- Arrange the compounds (1),(2) and (3) in their decreasing order of sta...
Text Solution
|
- CH(2)=CH-C-=C-CH=CH-CH(3)
Text Solution
|
- Mark out the most unlike form of polymerization of H(2)C=CH-CH=CH(2)
Text Solution
|
- The following reaction is a x,y-addition reaction. H(2)C=CH-CH=CH(2)+H...
Text Solution
|