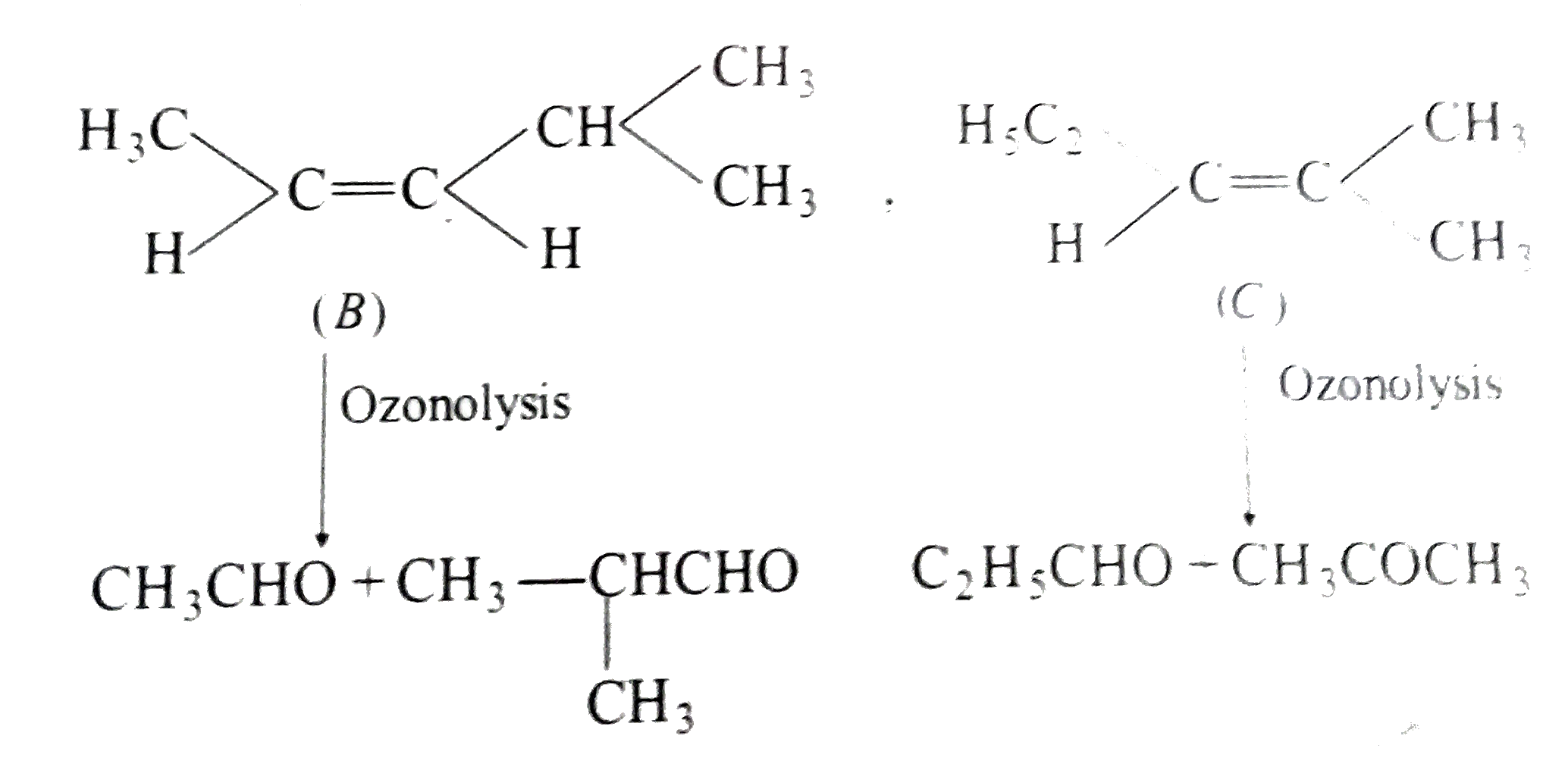
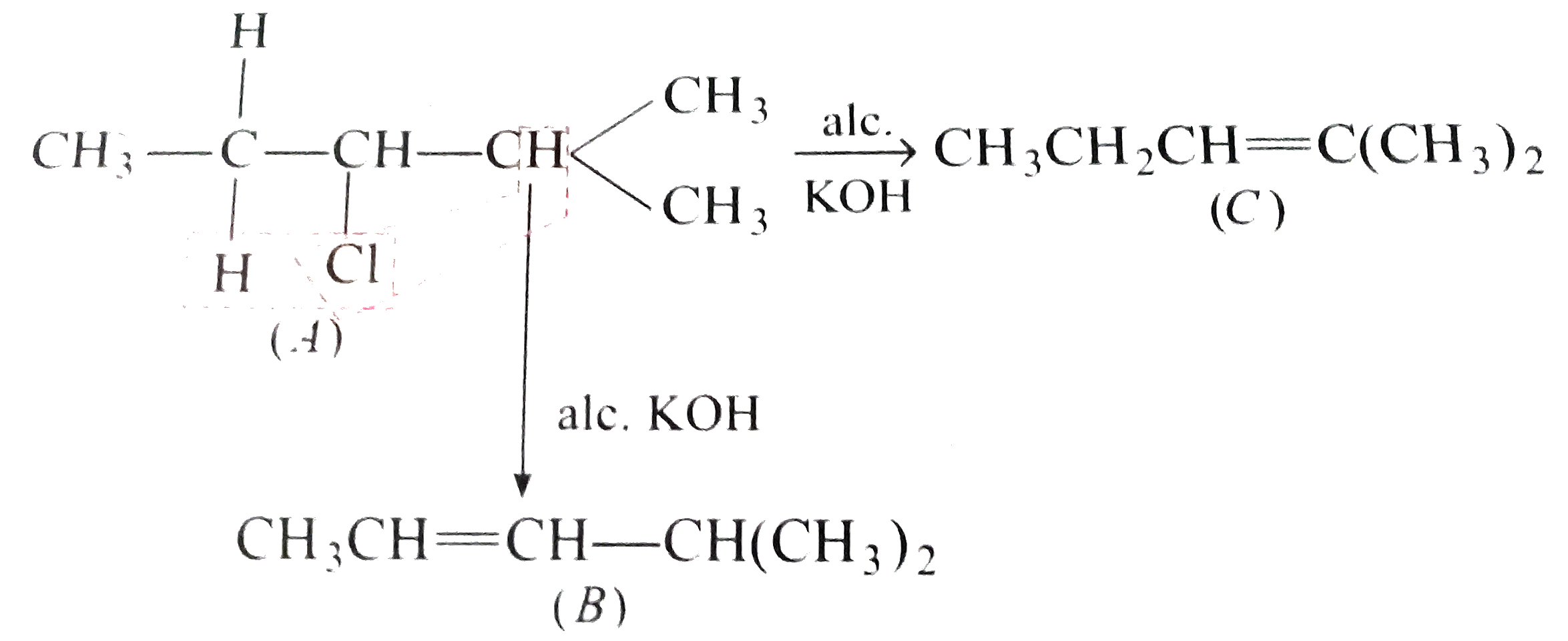
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OP TANDON-PROBLEMS BASED UPON STRUCTURES AND REACTIONS OF ORGANIC COMPOUNDS -Some Solved Problems
- A white precipitate was formed slowly when silver nitrate was added to...
Text Solution
|
- A chloro compound (A) showed the following properties : (i) decolour...
Text Solution
|
- From analysis and molecular weight determination the molecualr fomula ...
Text Solution
|
- Alkene (A) and (B) yield the same alcohol (C) on hydration. On vigoro...
Text Solution
|
- An acidic compound (A) , C4H8O3 , loses its optical activity on strong...
Text Solution
|
- A hydrocarbon A, of C8H10, on ozonolysis gives compound (B), C4H6O2 on...
Text Solution
|
- 1,4-pentadiene reacts with excess of HCI in the presence of benzoyl p...
Text Solution
|
- A mixture of an acid anhydride (A) and a mono basic acid (B) on heati...
Text Solution
|
- Hydrocarbon (A), C6H10 on treatment with H2//Ni,H2/Lindler catalyst an...
Text Solution
|
- An ester (A) (C4H8O2) , on treatment with excess methyl magnesium chlo...
Text Solution
|
- Two isomeric forms of an organic compound (A), C11H13OCI readily decol...
Text Solution
|
- One mole of the compound (A), C8H12 incapable of showing steroisomeris...
Text Solution
|
- A hydrocarbon (A) of the formula , C7H12 on ozonolysis gives a compoun...
Text Solution
|
- An organic compound (A), C18H20O on ozonolysis gives (B), C10H12O and ...
Text Solution
|
- An aldehyde (A) (C11H8O) , which does not undergo self aldol condensat...
Text Solution
|
- Catalytic dehydrogenation of methyl cyclohexane, obtained from petrole...
Text Solution
|
- Compound (A) (C8H8O) on treatment with NH2OH.HCI gives (B) and (C) rea...
Text Solution
|
- A Grignard reagent (A) and a haloalkene (B) react together to give (C)...
Text Solution
|
- An organic compound [A] C(8)H(6) on reacting with dilute sulphuric aci...
Text Solution
|
- Compound A of molecular formula C(9)H(7)O(2)Cl exists is keto form and...
Text Solution
|