A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
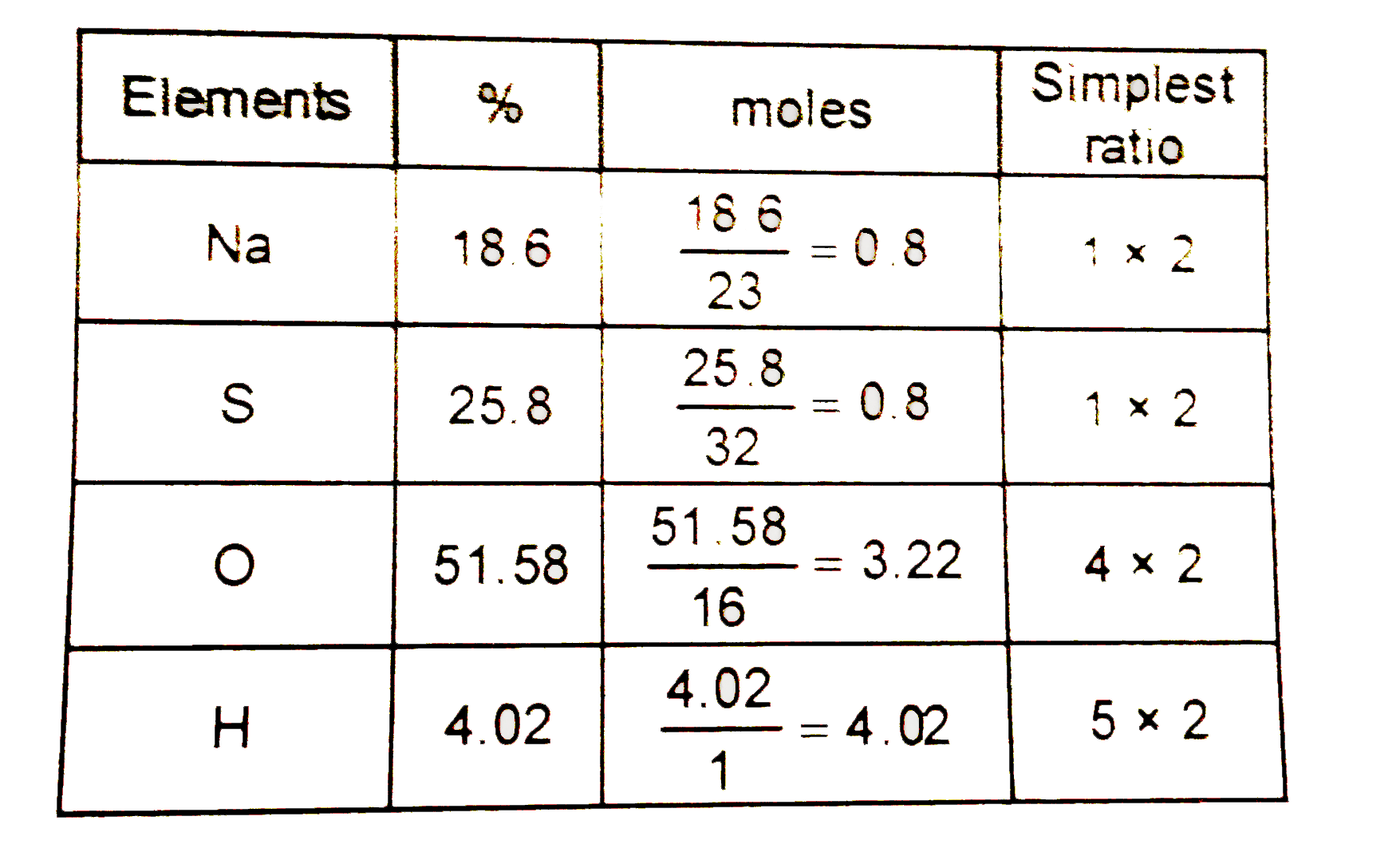
- A compound has the following composition by weight , Na = 18.60 %, S =...
Text Solution
|
- A compound has the following composition by weight , Na = 18.60 %, S =...
Text Solution
|
- An organic compound has the following percentage composition , C = 48 ...
Text Solution
|
- A crystalline compound when heated become anhydrous by losing 51.2 % o...
Text Solution
|
- Compound 'A' is found to contain 36.5 % Na,25.4 % S and 38.1 % O . Its...
Text Solution
|
- एक यौगिक की प्रतिशत रचना निम्नलिखित है- हाइड्रोजन =2.48% गन्धक =39....
Text Solution
|
- एक यौगिक का प्रतिशत संघटन निम्नलिखित है- C=40.6%,H=6.6%तथा O=52.8% ...
Text Solution
|
- A crystalline compound when heated became anhydrous by losing 51.2 % o...
Text Solution
|
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|