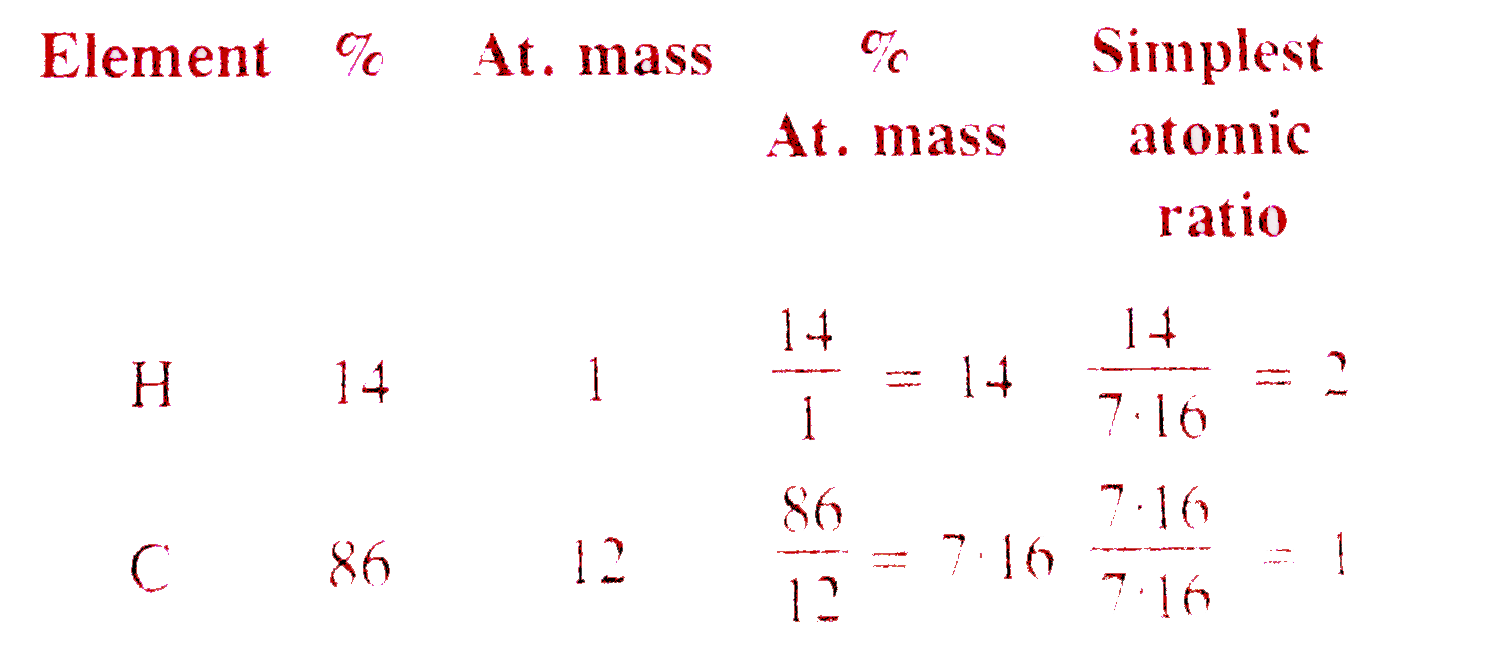A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise REVISION QUESTIONS FROM COMPETITIVE EXAMS|150 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRIGHT OBJECTIVE TYPE MCQs|42 VideosSOLUTIONS
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|10 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY-ULTIMATE PREPARATORY PACKAGE
- An unsaturated hydrocarbon weighing 1.68 g has a volume of 488 mL at S...
Text Solution
|
- A flask contains 2.0xx10^(13) molecules of CO(2). To this 1.5xx10^(14)...
Text Solution
|
- A flask contains 3.0xx10^(16) atoms of He. From This 6.6xx10^(15) atom...
Text Solution
|
- If one mole of rupees is distributed equally amongst all the poputlati...
Text Solution
|
- Polyethene can be produced from calcium carbide according to the follo...
Text Solution
|
- If law of conservation of mass holds good, 2.00 g of Na(2)SO(4) will r...
Text Solution
|
- If atomic mass of carbon was set at 100 u, what would be the value of ...
Text Solution
|
- A borane on analysis was found to contain 88.45% boron. Its empirical ...
Text Solution
|
- A sample of pure compound contains 2.04 g of sodium, 2.65xx10^(22) ato...
Text Solution
|
- A purified cytochrome protein was found to contain 0.376 % iron. What ...
Text Solution
|
- A purified pepsin was subjected to amino acid analysis. The amino acid...
Text Solution
|
- A peroxidase enzyme isolated from red blood cells was found to contain...
Text Solution
|
- A sample of hydrolysed potato starch is found to contain 0.086% phosph...
Text Solution
|
- Manganese forms non-stoichiometric oxides having the gereral formula f...
Text Solution
|
- Before 1961, an atomic mass unit scale was used whose basis was an ass...
Text Solution
|
- At one time, there was a atomic mass scale on the assignment of the va...
Text Solution
|
- A flask contains 10^(20) atoms of He (At. Mass =4) at S.T.P. (760 mm H...
Text Solution
|
- Eq. mass of A(x)B(y) is
Text Solution
|
- According to Dulong and Petit's rule, in case of solid elements. App...
Text Solution
|
- Out of atomic mass, mass number and atomic number, the physical quanti...
Text Solution
|
- Law of constant composition doesnot hold good for
Text Solution
|