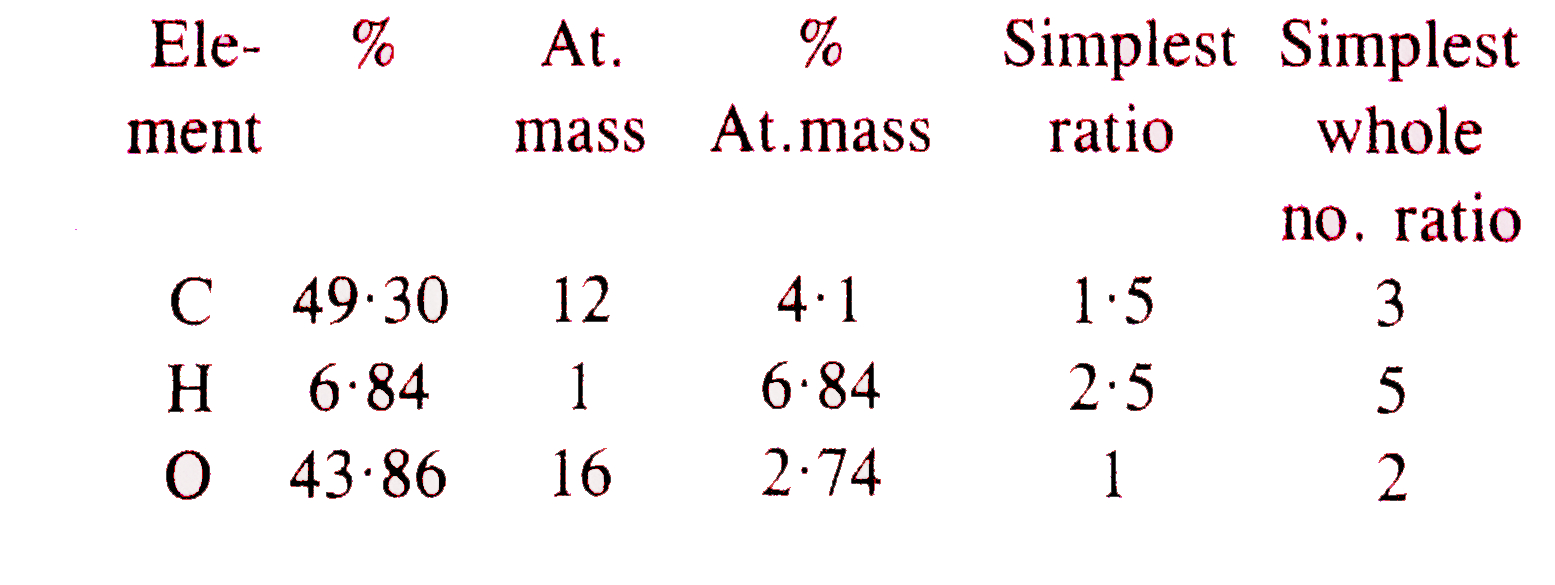A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRIGHT OBJECTIVE TYPE MCQs|42 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise LINKED COMPREHENSION TYPE MCQs|12 VideosSOME BASIC CONCEPTS OF CHEMISTRY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|20 VideosSOLUTIONS
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|10 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQs)|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY-REVISION QUESTIONS FROM COMPETITIVE EXAMS
- In a mole of water vapours at STP, the volume actually occupied or tak...
Text Solution
|
- Complete combustion of 0.858 g of compound X gives 2.63 g of CO(2) and...
Text Solution
|
- An organic compound contains 49.3% carbon. 6.84% hydrogen and its vapo...
Text Solution
|
- In the following reaction, which choice has value twice that of the eq...
Text Solution
|
- Vapour density of a gas is 22. What is its molecular mass?
Text Solution
|
- The empirical formula of an acid is CH(2)O(2), the probable molecular ...
Text Solution
|
- 1.5 mol of O(2) combine with Mg to form oxide MgO. The mass of Mg (at ...
Text Solution
|
- The mass of 112 cm^(3) of CH(4) gas at STP is
Text Solution
|
- The volume of water to be added to 100 cm^(3) of 0.5 NH(2)SO(4) to get...
Text Solution
|
- The reaction of calcium with water is represented by the equation Ca...
Text Solution
|
- Among the following pairs, the one which illustrates the law of multip...
Text Solution
|
- Mixture of sand and sulphur may best be separated by
Text Solution
|
- The set of numerical coefficients that balances the chemical equation ...
Text Solution
|
- 0.126 g of acid required 20 mL of 0.1 N NaOH for complete neutralisati...
Text Solution
|
- Which law directly explains the law of conservation of mass?
Text Solution
|
- Molarity of liquid HCl with density equal to 1.17g/cc is
Text Solution
|
- The modern atomic weight scale is based on
Text Solution
|
- The prefix 10^(18) is
Text Solution
|
- Difference in density is the basis of
Text Solution
|
- A mixture of sand and iodine can be separated by
Text Solution
|