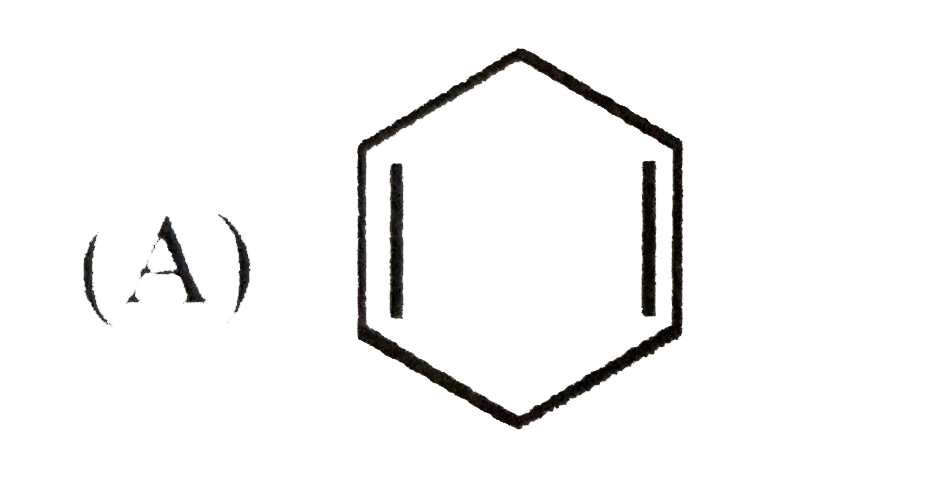
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise REVISION QUESTIONS FROM COMPETITIVE EXAMS.|192 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|64 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 Videos
Similar Questions
Explore conceptually related problems