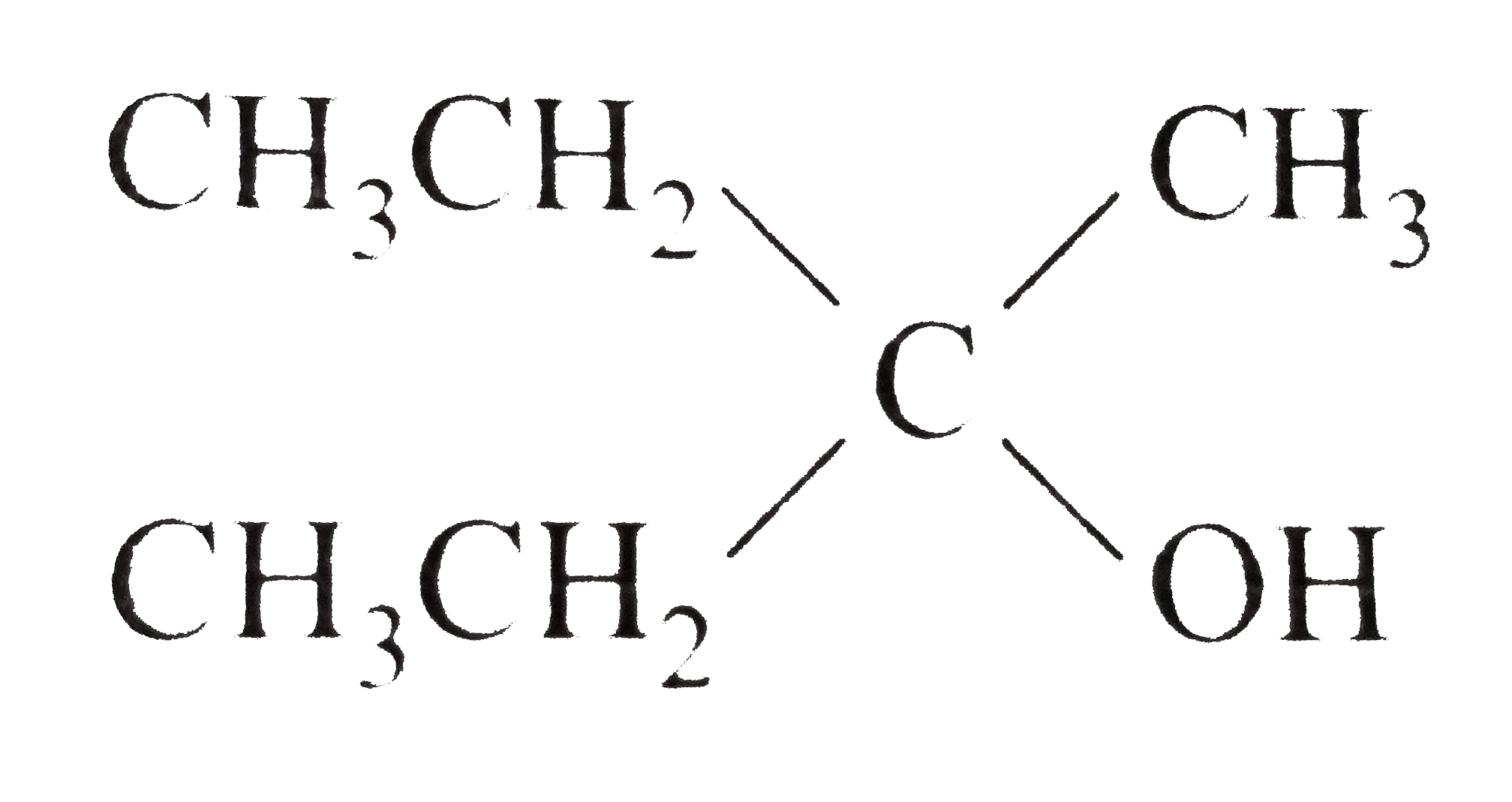
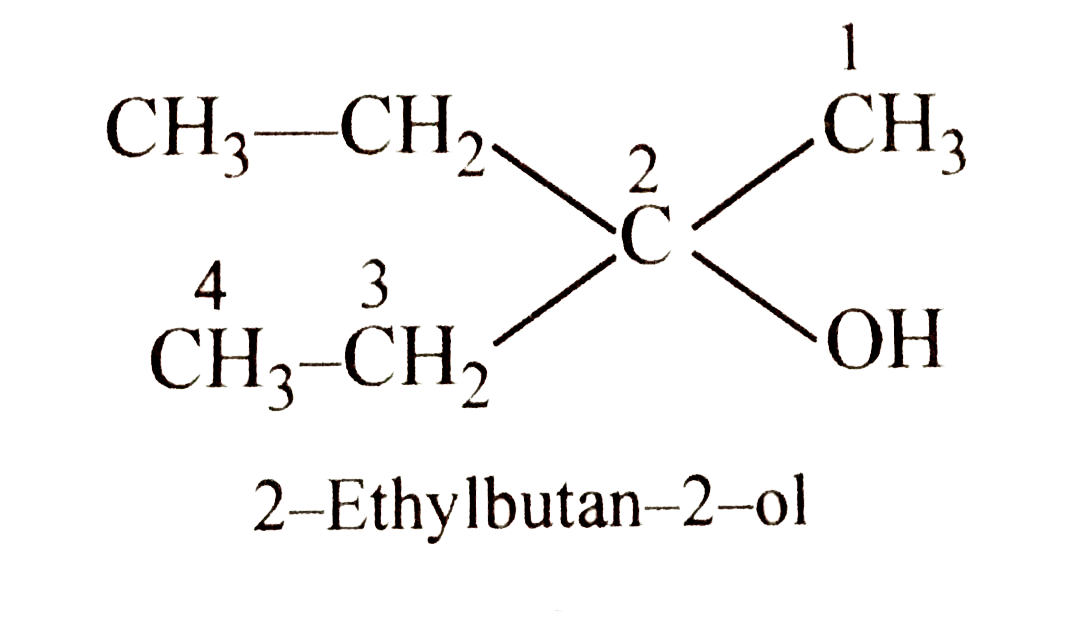A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|64 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise LINKED COMPREHENSION TYPE MCQS|10 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise Matrix Type Questions|2 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-BASIC PRINCIPLES OF ORGANIC CHEMISTRY-REVISION QUESTIONS FROM COMPETITIVE EXAMS.
- The IUPAC name of 4-isopropyl-m-xylene is
Text Solution
|
- IUPAC nomenclature of the given organic compound (CH(3))(2)C(CH(2)CH(3...
Text Solution
|
- The correct nomenclature (IUPAC) for the following alcohol is
Text Solution
|
- Which of the following compound has wrong IUPAC name?
Text Solution
|
- The name of Cl-CH(2)-underset(Br)underset(|)(C)= underset(Br)underset(...
Text Solution
|
- The IUPAC name of the following compound CH(3)-C(CH(3))(2)-CH=C(CH(3...
Text Solution
|
- IUPAC name of 3-isopropyl-o-oxylene is
Text Solution
|
- Name of the compound given below is
Text Solution
|
- Decreasing order C-C bond length is (I) C(2)H(4) , (II) C(2)H(2) (II...
Text Solution
|
- IUPAC name of CH(2)=CH-CH(CH(3)CH(2))underset(Br)underset(|)C=CH(2) is
Text Solution
|
- The IUPAC name of CH(3)COCH(CH(3))(2) is
Text Solution
|
- The IUPAC name of
Text Solution
|
- The IUPAC name of the following compound
Text Solution
|
- The names of some compounds are given. Which one not in the IUPAC syst...
Text Solution
|
- Pick out the most stable carbonium ion:
Text Solution
|
- Electrophilic reagents are
Text Solution
|
- Heterolytic cleavage of a covalent bond gives only,
Text Solution
|
- The reation CH(3)CH(2)Br+overset(-)OHrarrCH(3)CH(2)OH+Br^(-) is an...
Text Solution
|
- Which of the following contains three pairs of electrons in the valenc...
Text Solution
|
- Heterolysis of carbon-chlorine bond produces
Text Solution
|