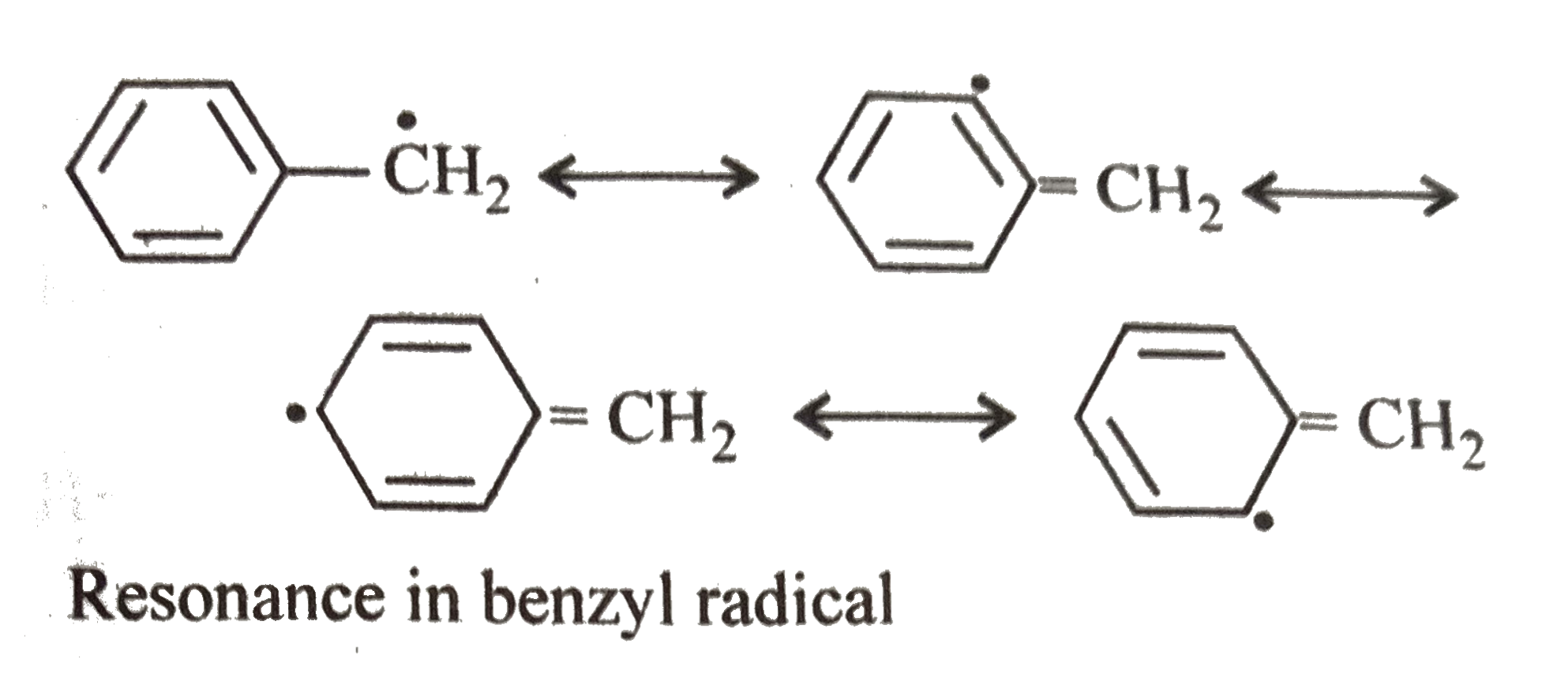
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|64 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise LINKED COMPREHENSION TYPE MCQS|10 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise Matrix Type Questions|2 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-BASIC PRINCIPLES OF ORGANIC CHEMISTRY-REVISION QUESTIONS FROM COMPETITIVE EXAMS.
- In the compound HC-=C-CH=CH2, the hybridizations of C-2 and C-3 carbon...
Text Solution
|
- Which of the following carbocations will be the most stable?
Text Solution
|
- Arrange the following free radicals in the order of decreasing stabili...
Text Solution
|
- Which isomer of hexane has only two different sets of structurally equ...
Text Solution
|
- The number of primary, secondary and tertiary carbons in 3,4-dimethylh...
Text Solution
|
- Which of the following is a 3 methyl butyl group.
Text Solution
|
- An example of electrophile is
Text Solution
|
- Which among the following free radicals is most stable
Text Solution
|
- The molecule which is free from angular strain is
Text Solution
|
- The reaction CH(2)=CH-CH(3)+HBrrarrCH(3)underset(Br)underset(|)CHCH(...
Text Solution
|
- IUPAC name is
Text Solution
|
- In which of the following species, all the three types of hybrid carbo...
Text Solution
|
- The correct IUPAC name of the acid is
Text Solution
|
- How many sigma(sigma) bonds are there in CH2=CH-CH = CH2 ?
Text Solution
|
- The common names of the lower fatty acids are obtained from
Text Solution
|
- How many sigma and pi bonds are present in the given compound Ph-CH=un...
Text Solution
|
- The IUPAC name of
Text Solution
|
- Which one of the following forms propanenitrile as the major product ?
Text Solution
|
- All carbon atoms sp^(2) hybridised in
Text Solution
|
- Hyperconjugation is most useful for stabilizing which of the following...
Text Solution
|