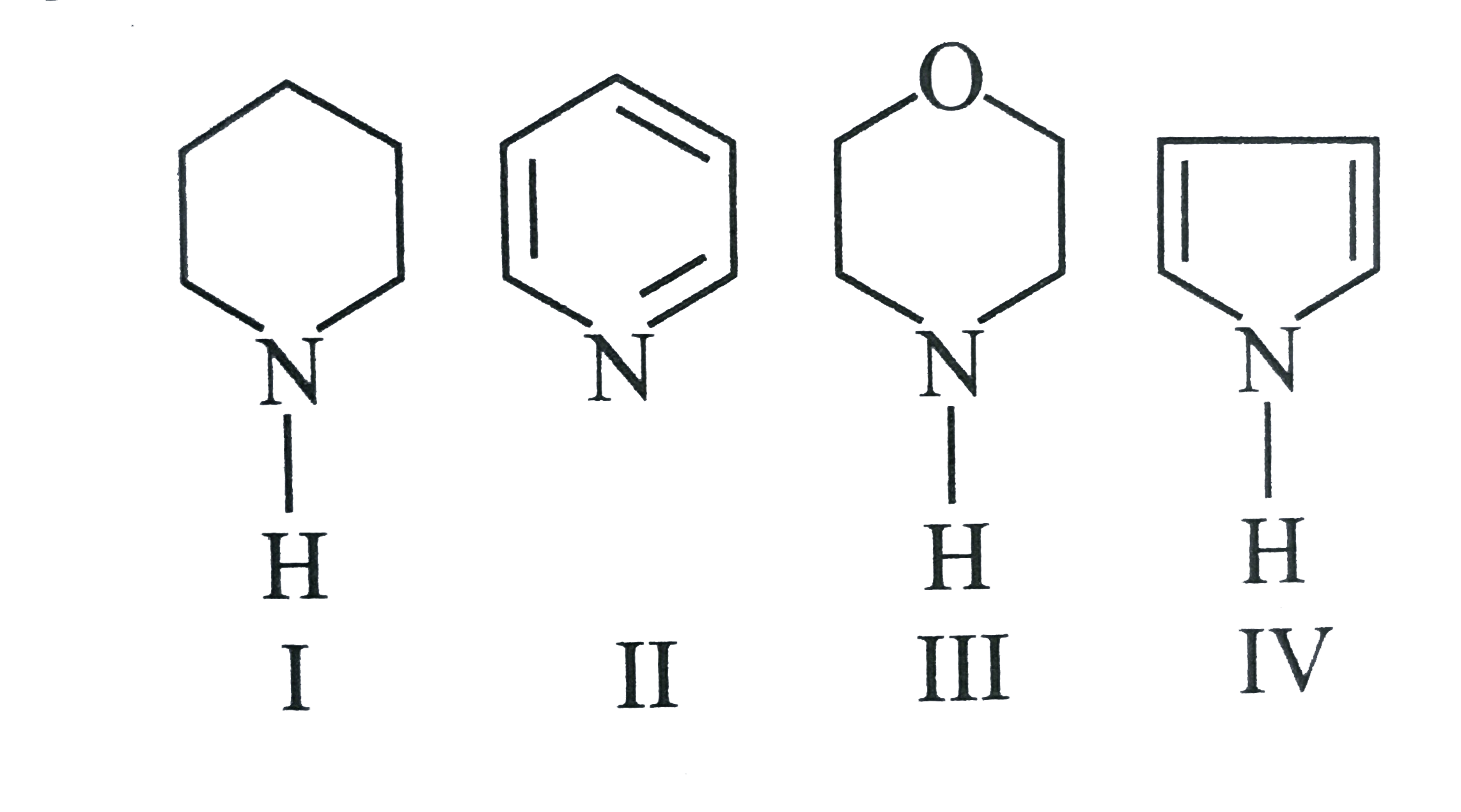
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise LINKED COMPREHENSION TYPE MCQS|10 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise REASON ASSERTION TYPE MCQS|12 VideosBASIC PRINCIPLES OF ORGANIC CHEMISTRY
DINESH PUBLICATION|Exercise REVISION QUESTIONS FROM COMPETITIVE EXAMS.|192 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 VideosBIOMOLECULES
DINESH PUBLICATION|Exercise MATRIX - MATCH TYPE|8 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-BASIC PRINCIPLES OF ORGANIC CHEMISTRY-SELECTED STRAIGHT OBJECTIVE TYPE MCQS
- The kind of delocalisation involving sigma bond orbitals is called………....
Text Solution
|
- The intermediate during the addition of HCl to propene in the presence...
Text Solution
|
- In the following compounds The order of basicity is
Text Solution
|
- The formation of cyanohydrin from ketone is an example of :
Text Solution
|
- Polarisation of electrons in acrolein may be written as :
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- Which of the following alkenes will react fastest with H(2) under cata...
Text Solution
|
- Which of the following has highest nucleophilicity ?
Text Solution
|
- The reaction of propene with HOCl proceeds via the addition of :
Text Solution
|
- Which is most stable ?
Text Solution
|
- Identify correct order of reactivity in electronphilic substitution re...
Text Solution
|
- Consider the following reaction: CH(3)underset(D)underset(|)CH-under...
Text Solution
|
- Which among the following is the most stable carbocation ?
Text Solution
|
- The names of some compounds are given. Which one not in the IUPAC syst...
Text Solution
|
- Due to the presence of an unpaired electron free radicals are
Text Solution
|
- The IUPAC name of C(6)H(5)COCl is
Text Solution
|
- IUPAC name of
Text Solution
|
- The general molecular formula, which represents the homologous series ...
Text Solution
|
- The increasing order of stability of the following free radicals is:
Text Solution
|
- The IUPAC name of the compound shown below is
Text Solution
|