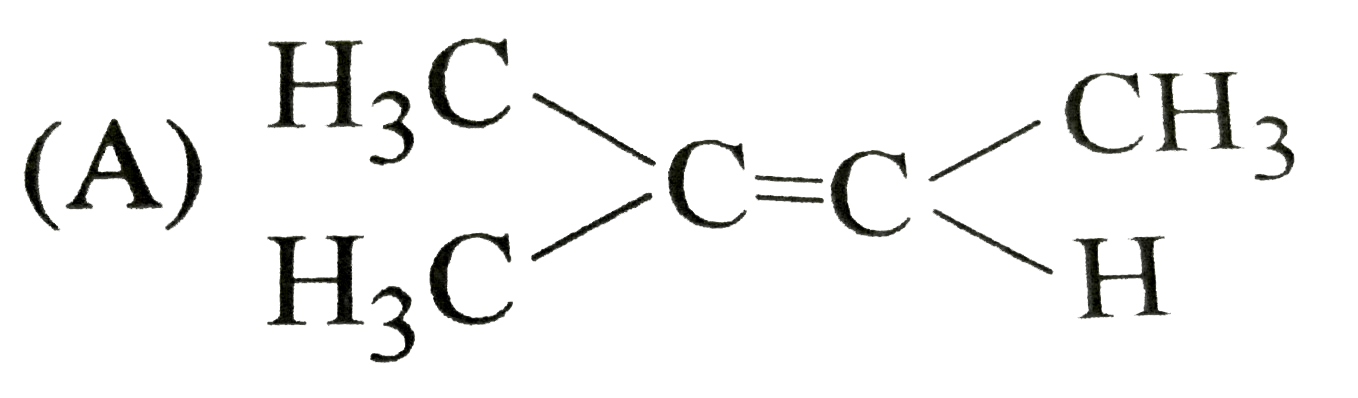
A
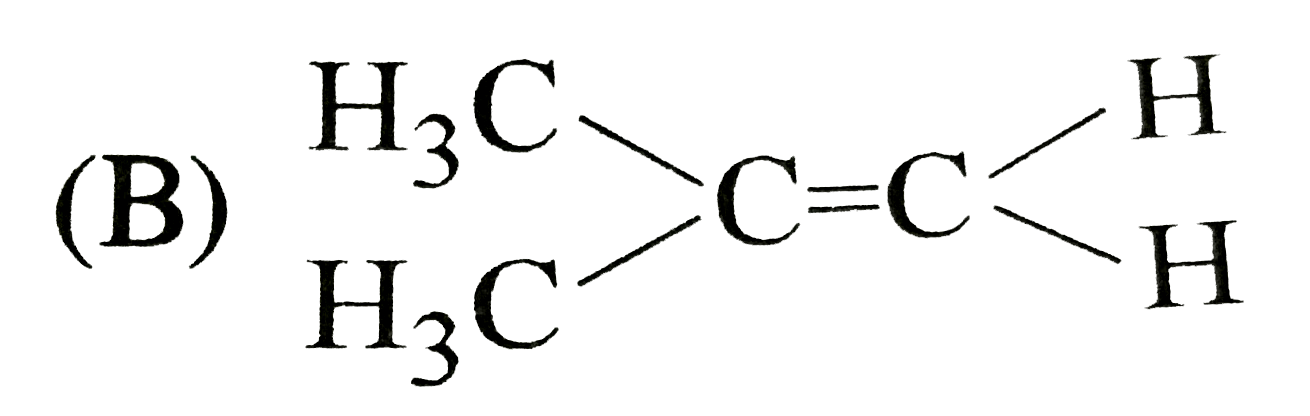
B
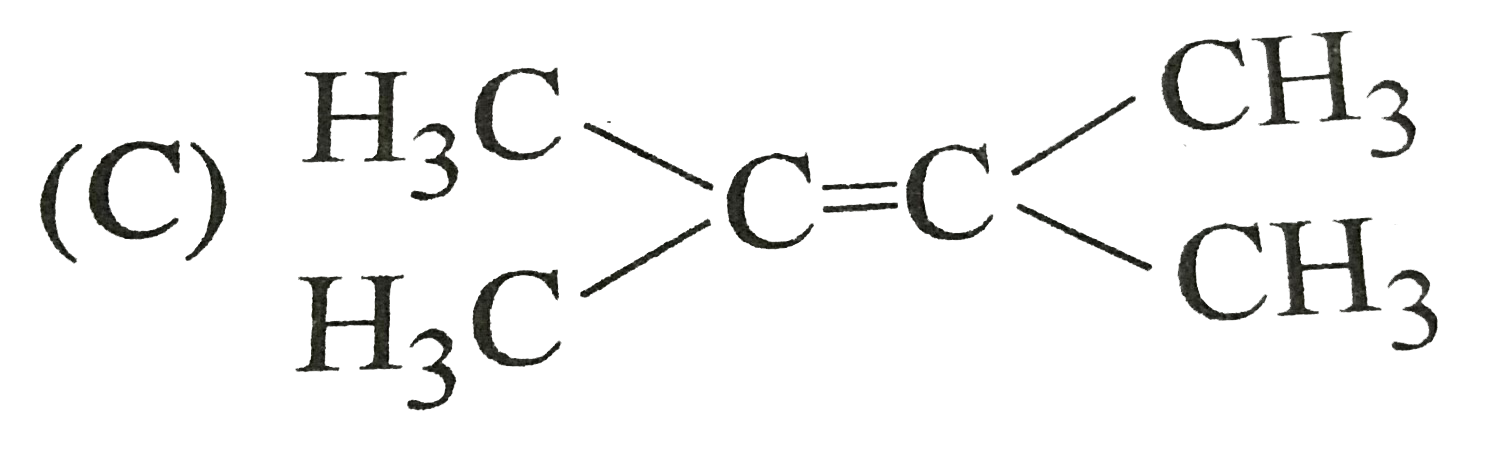
C
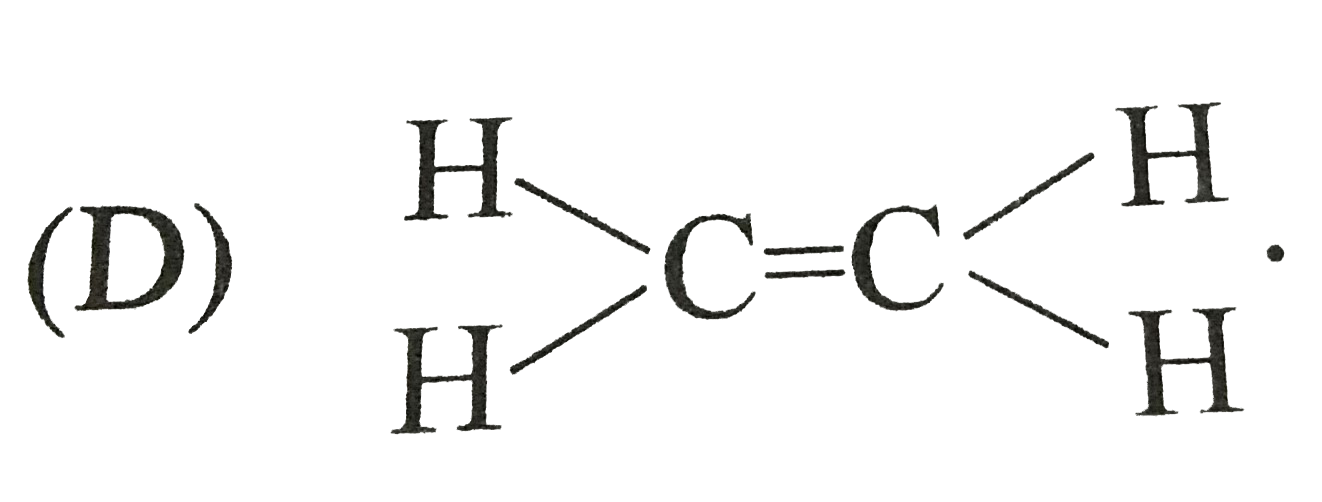
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-HYDROCARBONS-All Questions
- Westrosol has the following formula
Text Solution
|
- Ethylene combines with sulphur monochloride to form.
Text Solution
|
- The least reactive alkene towards hydrogenation is
Text Solution
|
- Ethyne+X overset(Ba^(2+))toProp-2-ene-nitrile. Here X can be
Text Solution
|
- Which of the following alkynes will not be able to show acidic charact...
Text Solution
|
- C+H(2) overset(3300K) to A overset(HCl)to B overset(HCl)to C In the ...
Text Solution
|
- Which of the following reagents give lewisite?
Text Solution
|
- A mixture of C(2)H(6), C(2)H(4) and C(2)H(2) is bubbled through alkali...
Text Solution
|
- Which of the following hydrocarbon is obtained by electrolysis of sodi...
Text Solution
|
- A metallic carbide on treatment with water gives a colouless gas which...
Text Solution
|
- Acetylene is used in the large scale production of
Text Solution
|
- The compounds A and B in the sequence CH-=CH+H(2)O overset(H(2)SO(4),...
Text Solution
|
- The ragent(s) required to convert 1-butyl to 2-butanone is (are)
Text Solution
|
- Which is the weakes acid among the following?
Text Solution
|
- CH-=CH+H(2)O overset(Hg^(+2))to CH(3)CHO The reation is known as
Text Solution
|
- Acetylene reacts with nitrogen in the presence of electric spark to pr...
Text Solution
|
- Propyne on passing through red hot copper tybe forms
Text Solution
|
- When CH(3)[CH(2)](3)C=CH is oxidised with hot alkaline KMnO(4) the pro...
Text Solution
|
- When but-2-yne is ozonised the product is
Text Solution
|
- What is X in the following reaction?
Text Solution
|