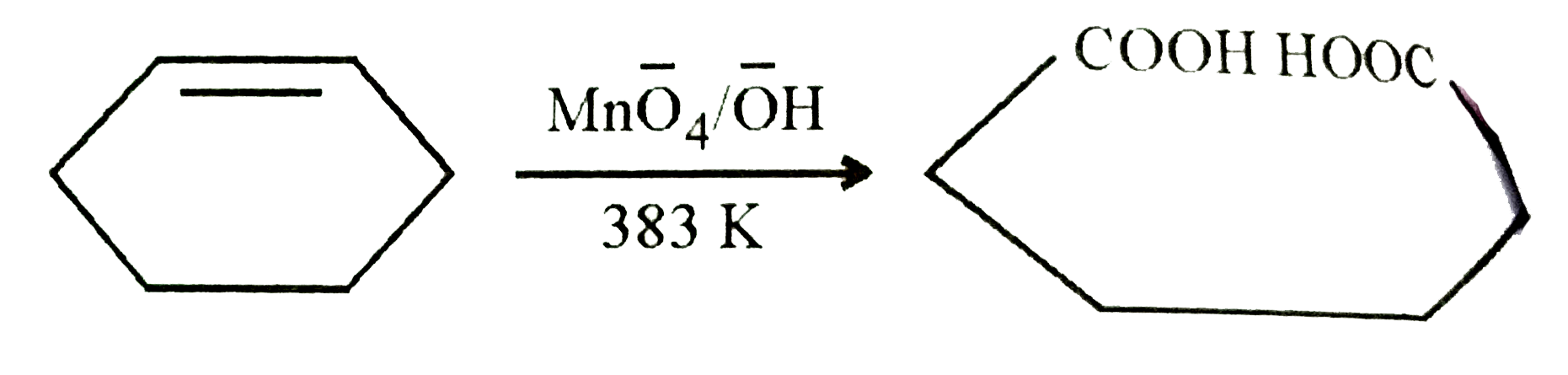A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-HYDROCARBONS-All Questions
- Hydroboratieon of 2-butyne followed by treatment with AcOH
Text Solution
|
- Which of the following is ketones cannot be formed by hydration of a s...
Text Solution
|
- A compound A with molecular formula C(6)H(10) on oxidation with hot KM...
Text Solution
|
- The Kolbe's electrolysis proceeds via
Text Solution
|
- The treatment of CH(3)OH "with" CH(3)Mgl releases 1.04mL of a gas at S...
Text Solution
|
- The structure of compound X in the following reaction is
Text Solution
|
- The product obtained on heating n-heptane with Cr(2)O(3),Al(2)O(3) at...
Text Solution
|
- Each of the following alkul halide reacts with sodium metal in presnce...
Text Solution
|
- Formation of butane by the action of zinc on ethyl bromide is known as
Text Solution
|
- The o,p-directing byt deactivating group is
Text Solution
|
- Which of the following carbocations is excepted to be most stable
Text Solution
|
- The function of CaO in the reaction RCO(2)^(-) underset("Heat")overs...
Text Solution
|
- Cyclohexene on ozonolysis yeilds
Text Solution
|
- Hydration of acetylene to ethanal is catalysed by
Text Solution
|
- The most reactive hydrocarbon is
Text Solution
|
- Propene, CH(3)-CH = CH(2), can be converted to 1-propanol by oxidation...
Text Solution
|
- Alkenes containing termincal double bond can be reduced by sodium in l...
Text Solution
|
- Benzene on ozolysis yields.
Text Solution
|
- If n is the number of carbon atoms in the potassium salt of a carboxyl...
Text Solution
|
- An alkene is formed from a carbocation by
Text Solution
|