A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Linked Comprehension Type MCQs|8 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Matrix Match Type MCQs|2 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Revision Questions From Competitive Exams|154 VideosNO IDEA
DINESH PUBLICATION|Exercise Unit Test -|1 VideosPOLYMERS
DINESH PUBLICATION|Exercise Matrix|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS -Selected Straight Objective Type MCQs
- Butanenitrile may be prepared by heating
Text Solution
|
- In the adddition of HBr to propene in the absence of peroxides, the fi...
Text Solution
|
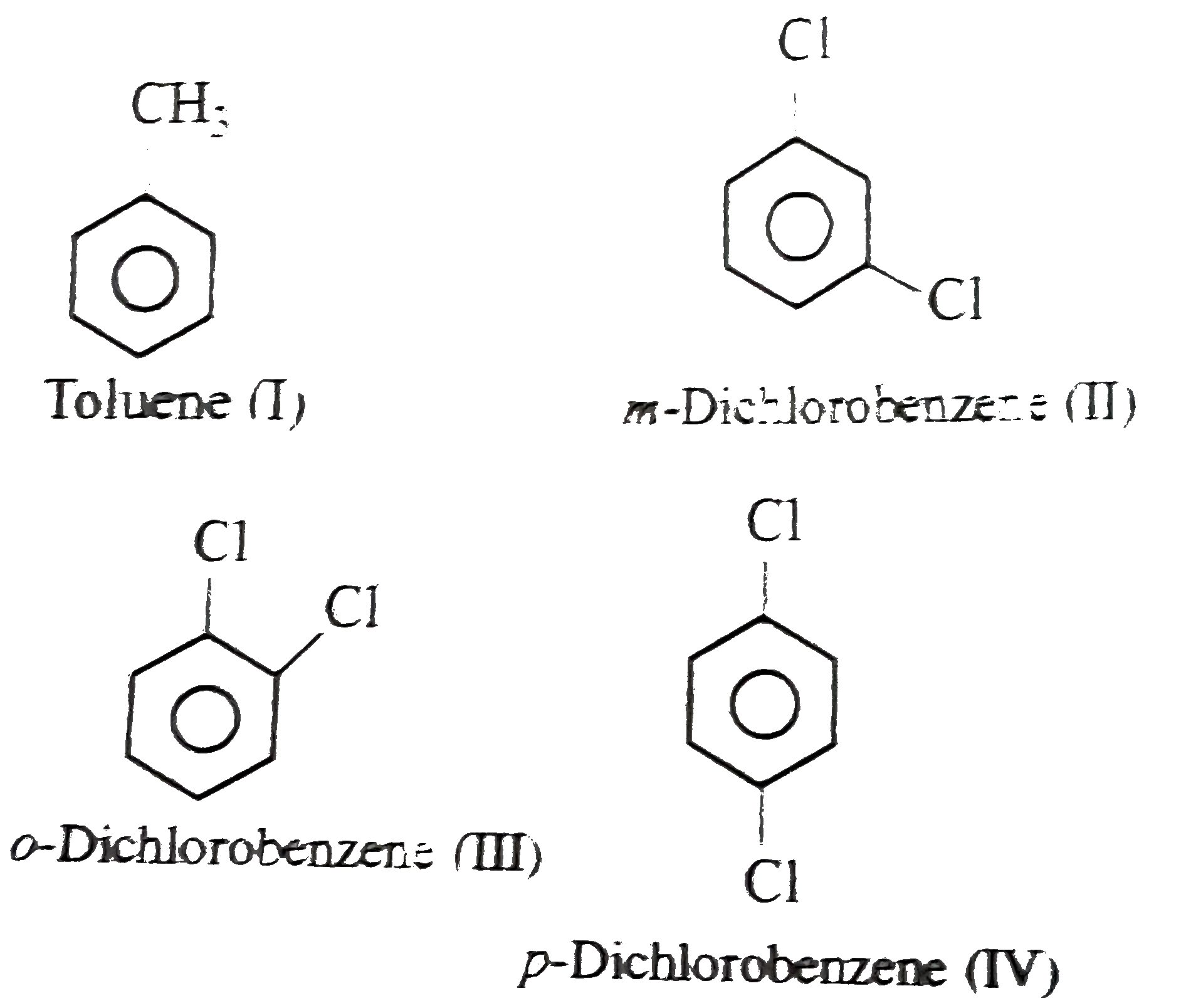
- Arrange the following compounds in order of increasing dipole moment ....
Text Solution
|
- The intermediate during the addition of HCl to propene in the presence...
Text Solution
|
- The number of possibel enanntiomer pairs that can be produced during m...
Text Solution
|
- During debromination of meso-dibromobutane, the major compound formed ...
Text Solution
|
- In the reaction of p-chlorotoluene with KNH(2) in liguid NH(3), the ma...
Text Solution
|
- (CH(3))(3)-C-MgCl on reaction with D(2)O produces
Text Solution
|
- Which of the following will react with water?
Text Solution
|
- A solution of (+)-2-chloro-2-phenyl ethane in toluene racemises slowly...
Text Solution
|
- The order of reactivities of the following alkyl halides for a S(N^(2)...
Text Solution
|
- Which of the following has the highest nucleophilicity ?
Text Solution
|
- The reaction of propene with HOCl proceeds via the addition of :
Text Solution
|
- As SN2 reaction at an asymmetric carbon of a compound always gives:
Text Solution
|
- The number of isomers for the compound with molecular formula C(2)BrCl...
Text Solution
|
- In the presence of peroxide, hydrogen chloride and hydrogen iodide do ...
Text Solution
|
- Identify the set of reagents // reaction conditions 'X' and 'Y' in the...
Text Solution
|
- Consider the following reaction : H(3)C-underset(D)underset(|)(C )H-u...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
Text Solution
|