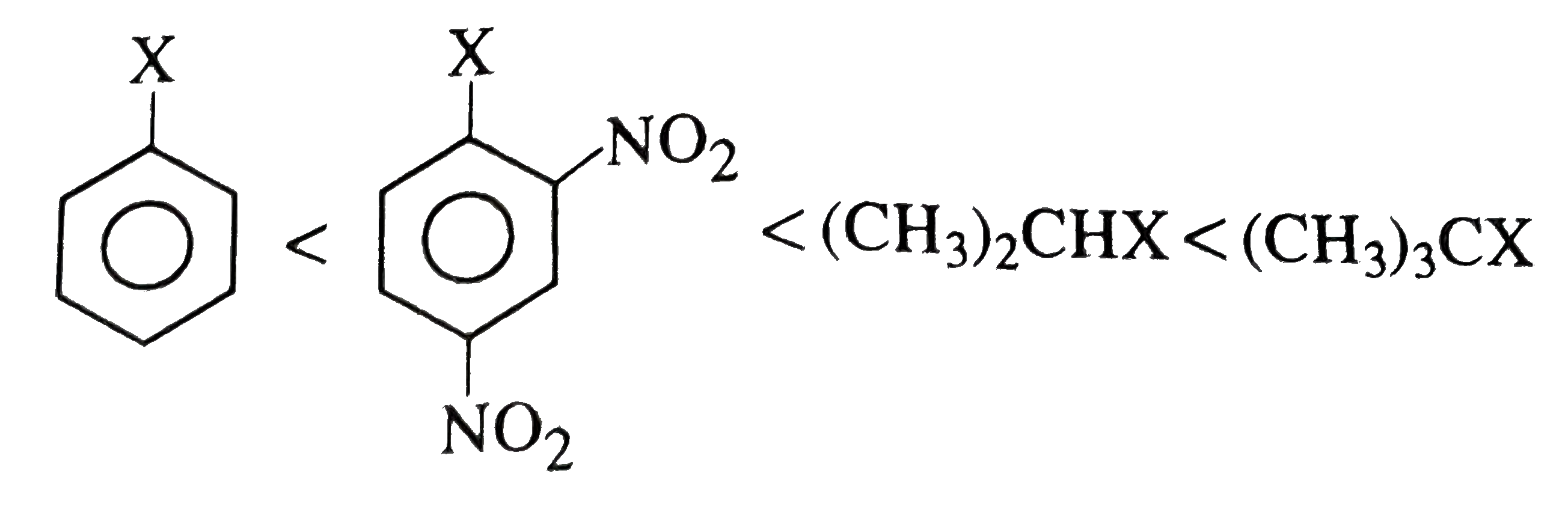
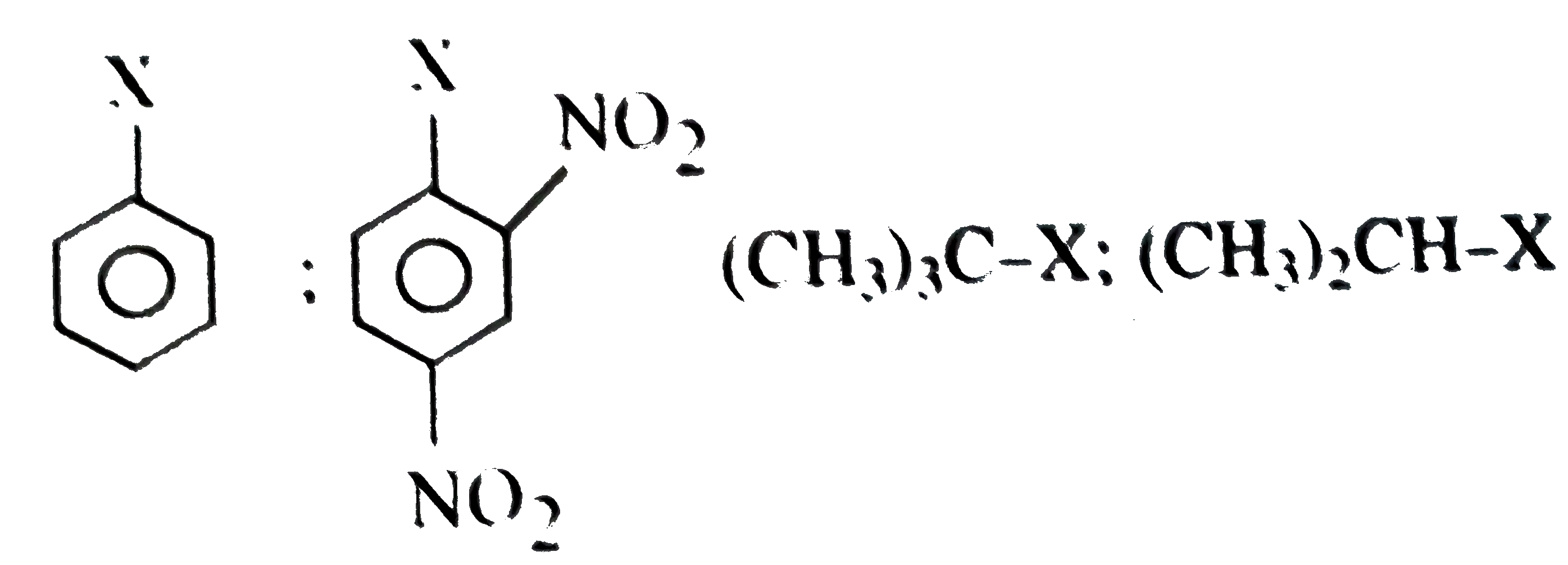A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Linked Comprehension Type MCQs|8 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Matrix Match Type MCQs|2 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise Revision Questions From Competitive Exams|154 VideosNO IDEA
DINESH PUBLICATION|Exercise Unit Test -|1 VideosPOLYMERS
DINESH PUBLICATION|Exercise Matrix|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS -Selected Straight Objective Type MCQs
- The IUPAC name of the compound shown below
Text Solution
|
- CH(3)Br+Nu^(-)rarrCH(3)Nu+Br^(-) The decreasing order of the rate of...
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory .
Text Solution
|
- The structure of the major product formed in the following reaction is...
Text Solution
|
- Trans-2-phenyl-1-bromocyclopentane on reaction with alcoholic KOH prod...
Text Solution
|
- Which of the following is not chiral?
Text Solution
|
- The number of stereoisomers obtained by bromination of trans-2-butene ...
Text Solution
|
- The reagents for the following conversion is/are
Text Solution
|
- Predict the product C obtained in the following reaction of butyne-1. ...
Text Solution
|
- For the following (i) I^(-) (ii) Cl^(-) (iii) Br^(-) the increasin...
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives predom...
Text Solution
|
- Which of the following is the correct order of decreasing S(N^(2)) rea...
Text Solution
|
- In a S(N)2 substitution reaction of the type R-Br+Cl^(-) overset("DM...
Text Solution
|
- H(3)C-underset(CH(3))underset(|)(CH)-CH=CH(2)+HBr to A. A is predomi...
Text Solution
|
- How many stereoisomerse does this molecule has? CH(3)CH=CHCH(2)CHBrC...
Text Solution
|
- The organic chloro compound, which shows complete stereochemical inver...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- What would be the produt formed when 1-bromo-3 chorocyclobutane reacts...
Text Solution
|
- Which one is most reactive towards S(N^(1)) reaction?
Text Solution
|
- The correct order of increasing reactivity of C-X bond towards nucleop...
Text Solution
|