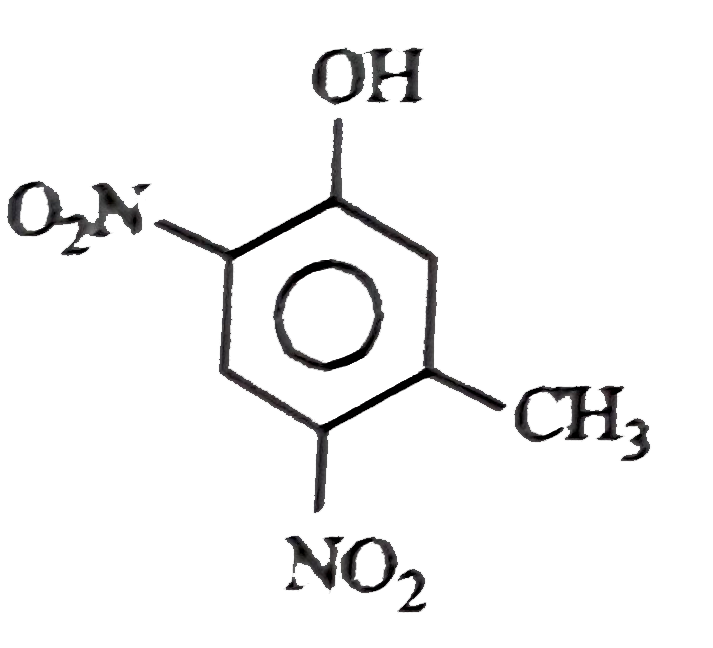
A
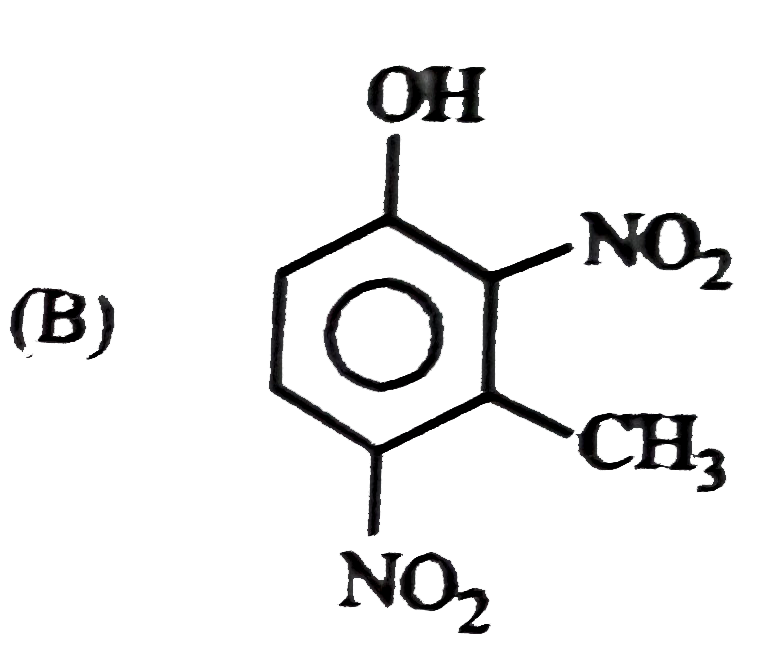
B
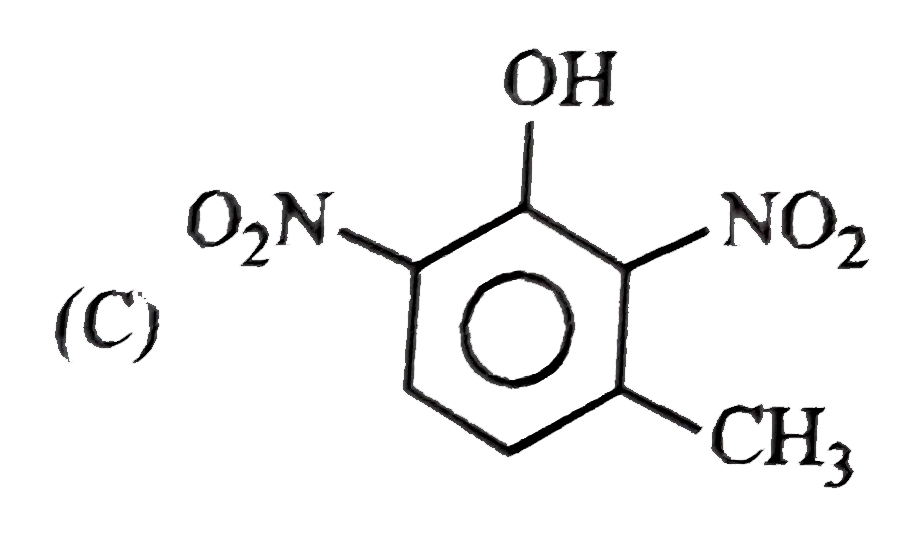
C
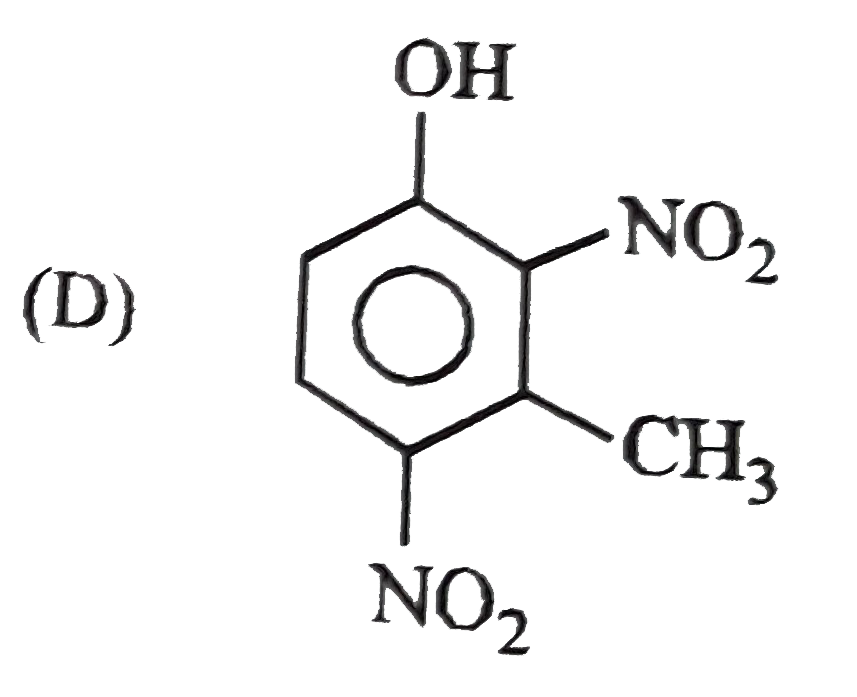
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
NO IDEA
DINESH PUBLICATION|Exercise Brain Teasers -34|1 VideosNO IDEA
DINESH PUBLICATION|Exercise Brain Teasers -35|1 VideosNO IDEA
DINESH PUBLICATION|Exercise Brain Teasers -32|1 VideosIONIC EQUILIBRIUM
DINESH PUBLICATION|Exercise UNIT TEST -2|30 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS
DINESH PUBLICATION|Exercise UNIT TEST - 5|37 Videos
Similar Questions
Explore conceptually related problems