A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
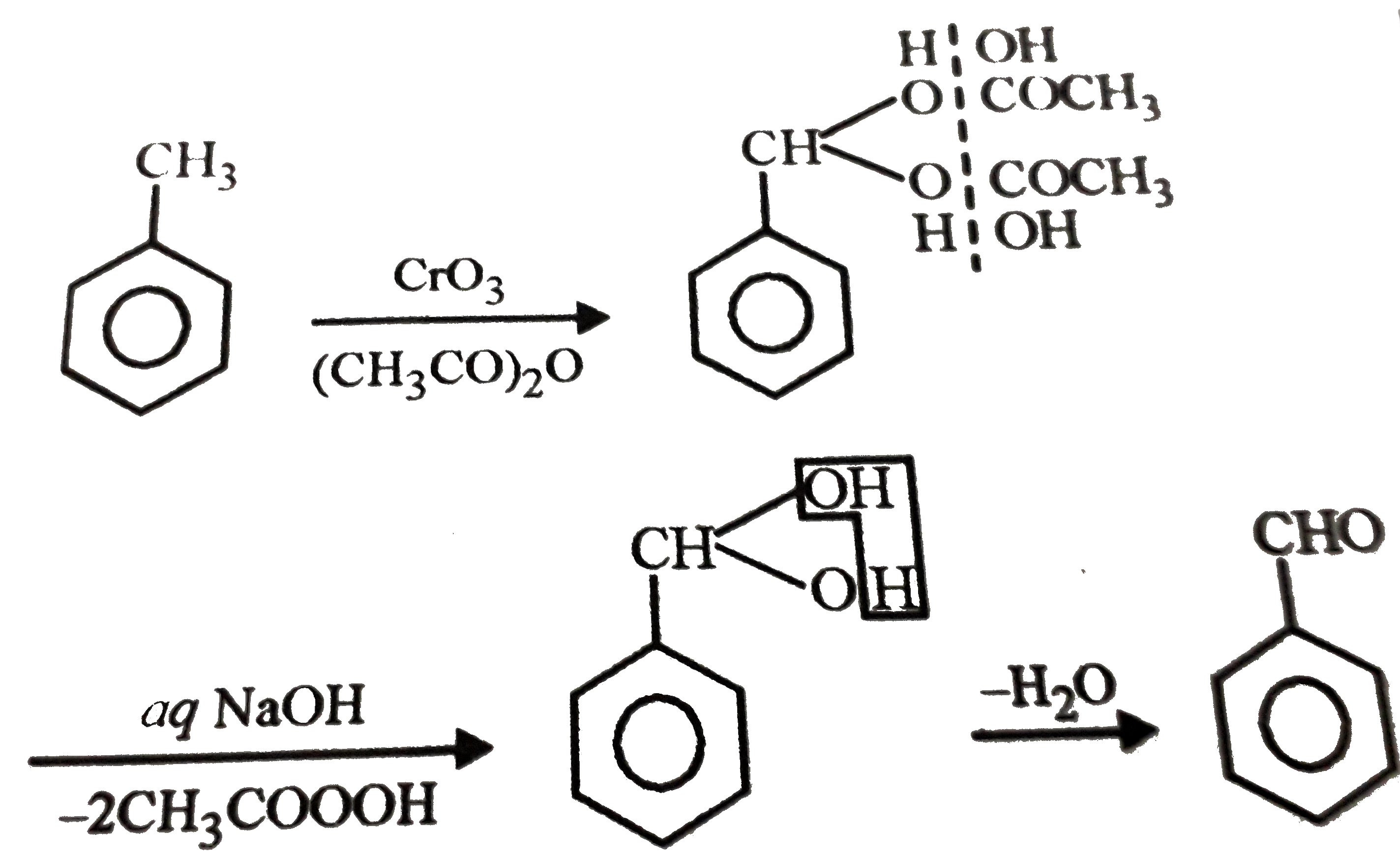
- Oxidation of toluene with CrO(3) in the presence of (CH(3)CO)(2)O give...
Text Solution
|
- CrO(3) on treatment with NaOH solution produces
Text Solution
|
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|
- Oxidation of toluene with CrO(3) in the presence of (CH(3)CO)(2)O give...
Text Solution
|
- Oxidation of toluene with CrO(3) in presence of (CH(3)CO)(2)O gives...
Text Solution
|
- Oxidation of toluene with CrO(3) in the presence of (CH(3)CO)(2)O give...
Text Solution
|
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|
- Phenol on treatment with CO(2) in the presence of NaOH followed by aci...
Text Solution
|
- टॉलूईन के CrO(3) तथा ऐसीटिक ऐनहाइड्राइड के द्वारा ऑक्सीकरण पर प्राप्त ...
Text Solution
|