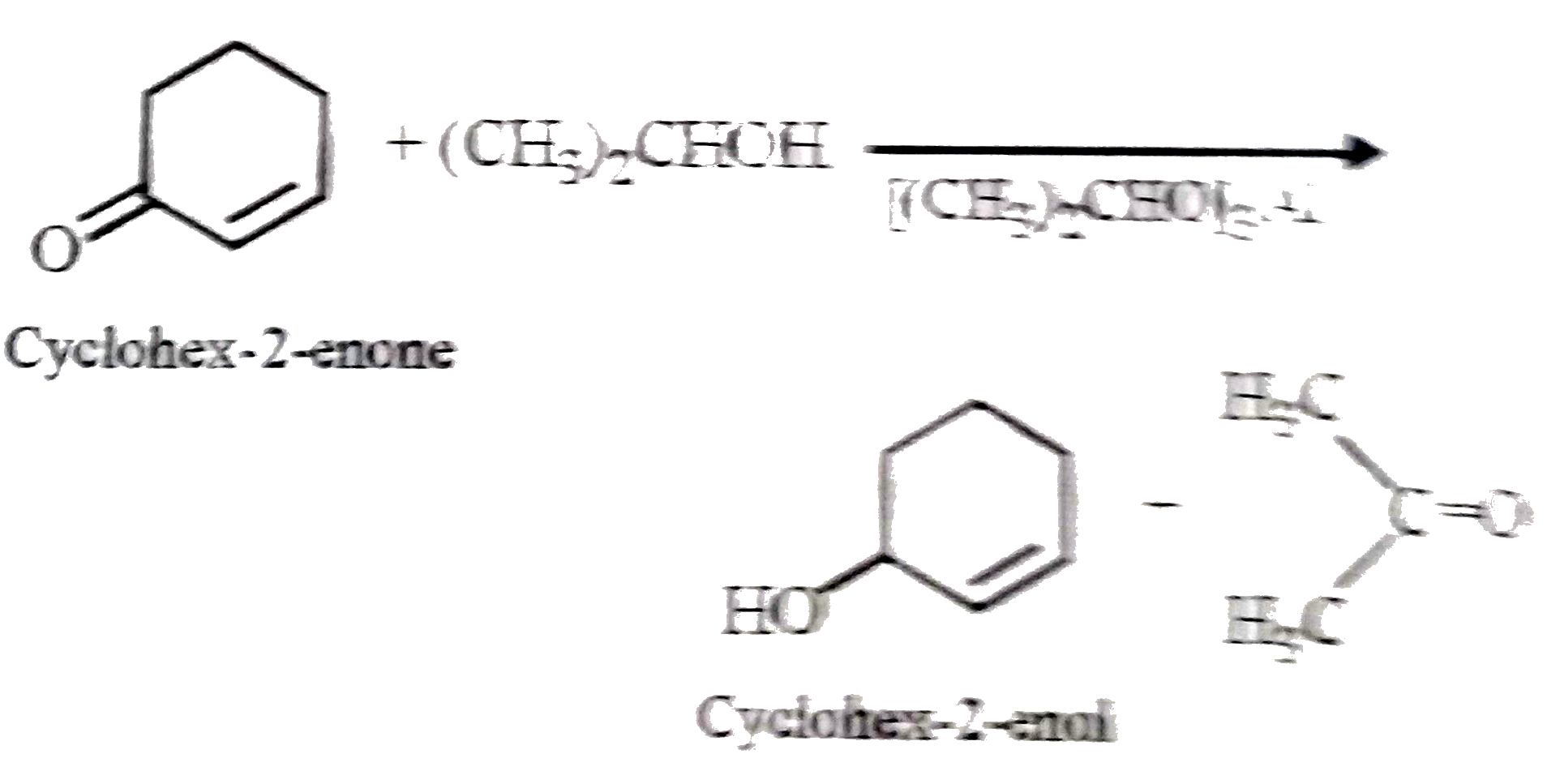
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Reduction with aluminium isopropoxide in excess of Isopropyl alcohol i...
Text Solution
|
- Which alcohol is prepared from the following ketones via MPV reduction...
Text Solution
|
- Complete the following MPV reductions:
Text Solution
|
- The catalyst and solvent used in MPV (Meerwein- Ponndorf-Verley) react...
Text Solution
|
- Reduction of aldehyde or ketone with aluminium isopropoxide to alcohol...
Text Solution
|
- Reduction with aluminium isopropoxide in excess of Isopropyl alcohol i...
Text Solution
|
- Reduction with aluminium isopropoxide in excess of Isopropyl alcohol i...
Text Solution
|
- Which alcohol is prepared from the following ketones via MPV reduction...
Text Solution
|
- METHODS OF PREPARATION OF ALCOHOLS|REDUCTION OF ALDEHYDES AND KETONES|...
Text Solution
|