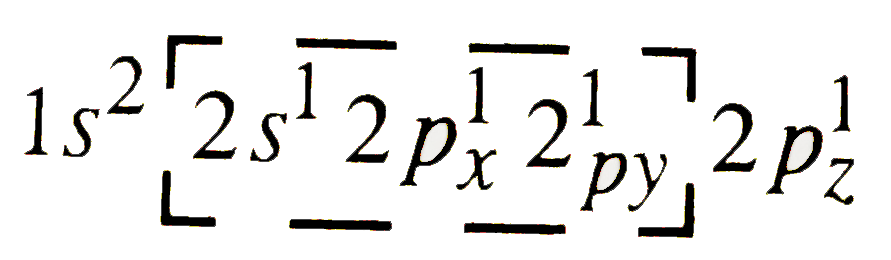A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE CARBON FAMILY
DINESH PUBLICATION|Exercise RQ|102 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Selected straight|31 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQs)|8 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE CARBON FAMILY-Ultimate
- The carbon atoms in graphite are
Text Solution
|
- Stannous oxide can be obtained by
Text Solution
|
- Tin (II) chloride (anhydrous) can be obtained
Text Solution
|
- Tin (IV) chloride (anhydrous) can be obtained
Text Solution
|
- Tin (II) fluoride (anhydrous ) can be obtained by
Text Solution
|
- Lead (IV) oxide is obtained by
Text Solution
|
- Oridinary sand (SiO(2)) is attacked by
Text Solution
|
- When a mixture of sand and KNO(3) is heated strongly the gaseous prod...
Text Solution
|
- Tin bronze is
Text Solution
|
- Lead sulphate dissolves in
Text Solution
|
- Which of the following is not an ore of lead ?
Text Solution
|
- The common blueing agent used for removing yellow tint from clothes (i...
Text Solution
|