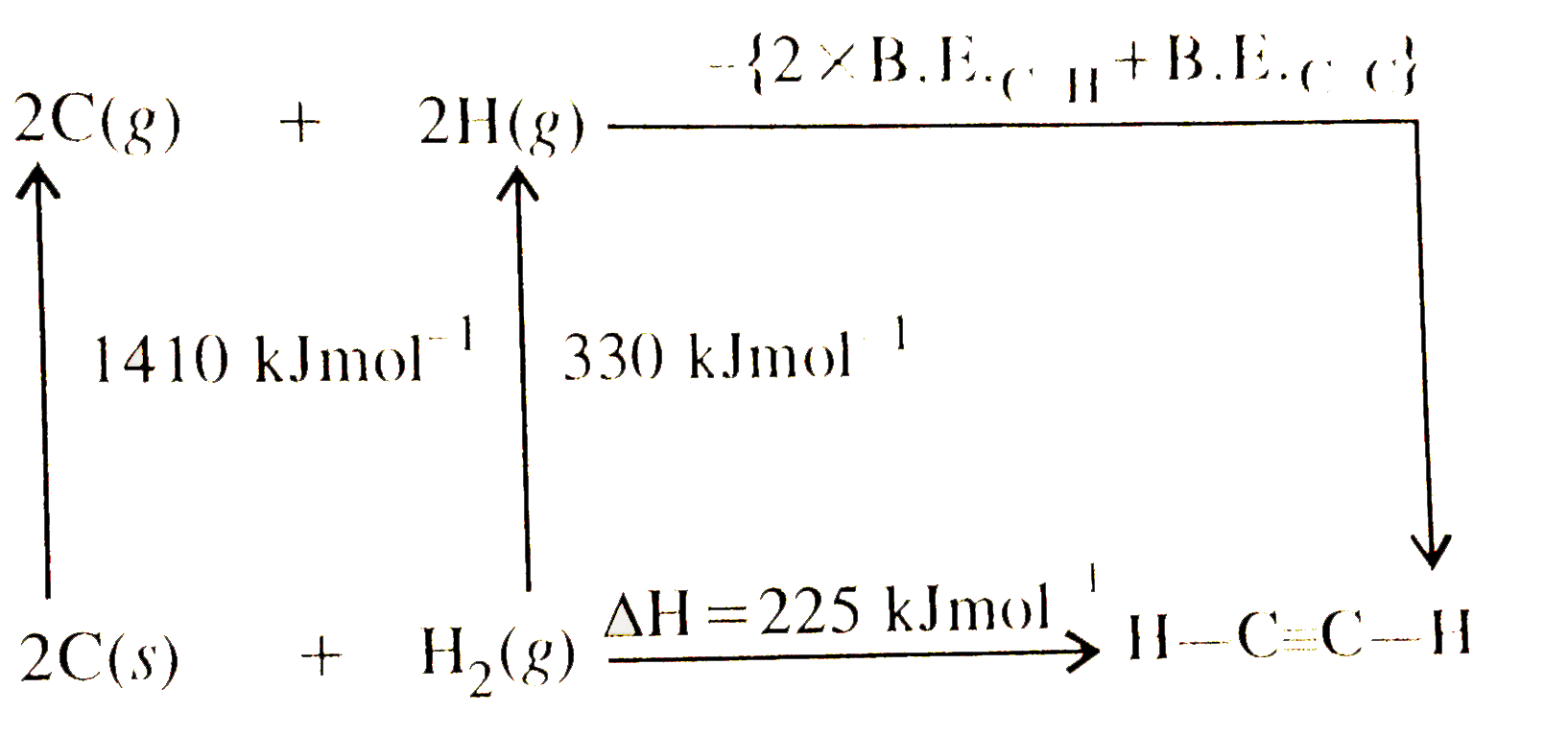A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE CARBON FAMILY
DINESH PUBLICATION|Exercise Selected straight|31 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Matrix|2 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Ultimate|11 VideosSURFACE CHEMISTRY
DINESH PUBLICATION|Exercise BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQs)|8 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE CARBON FAMILY-RQ
- Tungsten carbide is an example of
Text Solution
|
- SiO(2) is reacted with sodium carbonate. What is the gas liberated ?
Text Solution
|
- Which of the following oxides is amphoteric in nature?
Text Solution
|
- In silicon dioxide:
Text Solution
|
- Formula for tear gas is
Text Solution
|
- Which of the following is potassium ferricynide ?
Text Solution
|
- Sodium nitroprusside when added to an alkaline solution of sulphide io...
Text Solution
|
- White lead is
Text Solution
|
- Which of the following is combustion reactions ?
Text Solution
|
- vii. The stability of dihalides of Si, Ge , Sn and Pb increases stead...
Text Solution
|
- How many O-atoms are shared for SiO(4) tetrahedral in silicate anion o...
Text Solution
|
- Name the type of the structure of silicate in which one oxygen atom of...
Text Solution
|
- Which of the following on thermal decomposition yields a basic as well...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- With respect to graphite and diamond, which of the statements is (are ...
Text Solution
|
- The basic structural unit of silicates is
Text Solution
|
- Which of the following structure is similar to graphite e?
Text Solution
|
- The number of carbon atoms per unit cell of diamond unit cell is
Text Solution
|
- Which of these is not a monomer for a high-molecular mass silicone pol...
Text Solution
|
- Which of the following does not give oxygen on heating ?
Text Solution
|