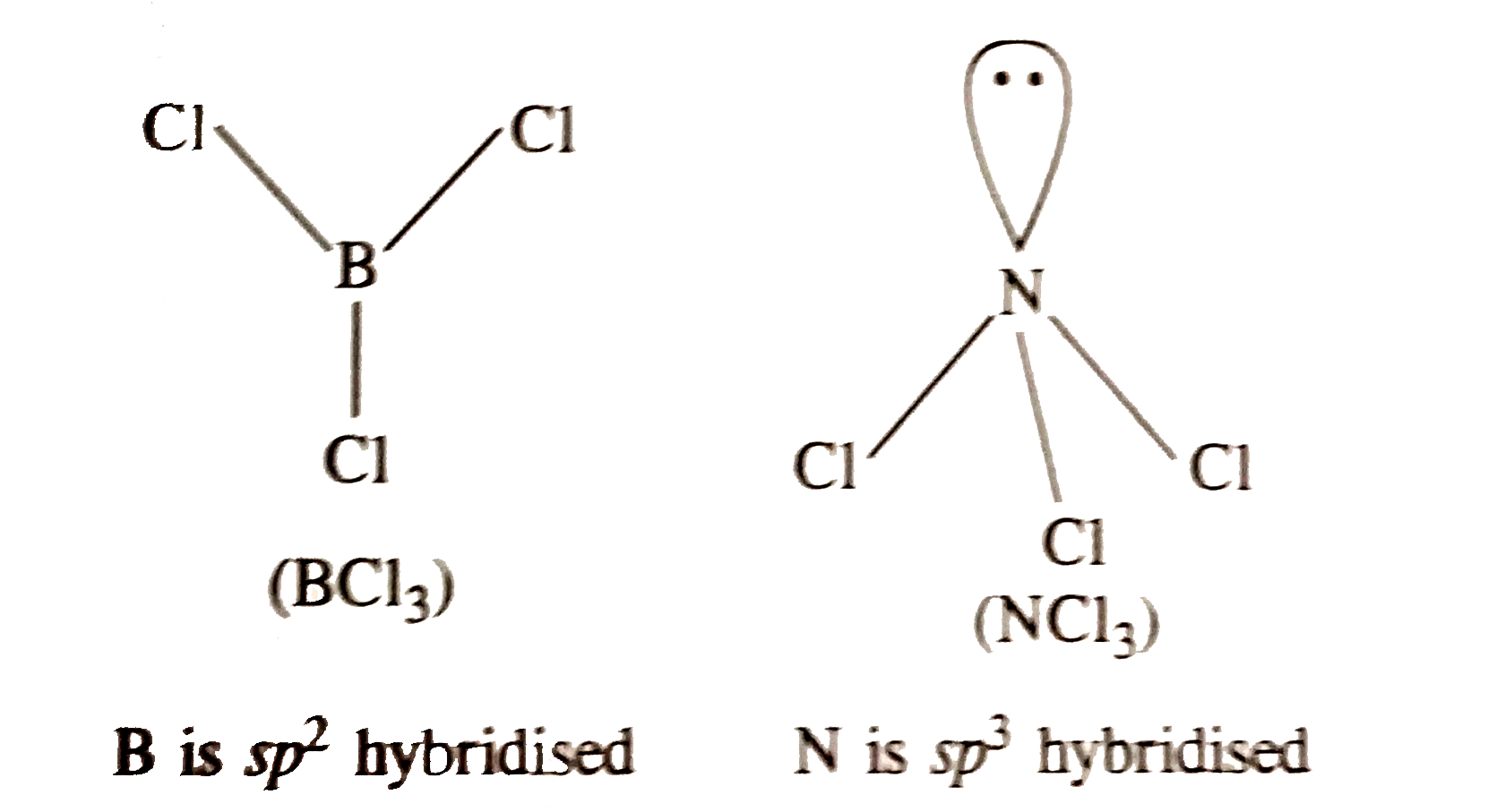A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Selected straight objective type|38 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Linked comprehension|3 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Ultimate|14 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Revision question
- Which of the following phosphorus is the most reactive?
Text Solution
|
- Which element of group 15 undergoes sublimation?
Text Solution
|
- The BCl3 is a polar molecule whereas NCl3 is pyramidal because
Text Solution
|
- The electronic configuration of an element is 1s^2 1s^2 2p^6 3s^2 3p^6...
Text Solution
|
- The most acidic of the following compounds is
Text Solution
|
- Which of the following halides is the most acidic?
Text Solution
|
- The acid which forms two series of salts is
Text Solution
|
- Which of the following is a cyclic phosphate?
Text Solution
|
- Which of the following has least covalent P-H bond
Text Solution
|
- The basic character of hydrides of the V-group elements decreases in ...
Text Solution
|
- At. No. of N is 7, the At. No. of the third member of nitrogen family ...
Text Solution
|
- Which of the following oxides will be the least acidic?
Text Solution
|
- the correct structural formula of hypophosphorous acid is
Text Solution
|
- Which of the following compounds does not exist ?
Text Solution
|
- In Nitrogen family the H-M-H angle in the hydrides MH(3) gradually bec...
Text Solution
|
- In PO(4)^(3-) the formal charge on each O-atom and P-O bond order resp...
Text Solution
|
- P-O-P bond is present in
Text Solution
|
- Which of the following is the correct statement for PH3?
Text Solution
|
- The equivalent weight of phosphoric acid (H3 PO4) in the reaction
Text Solution
|
- Boiling/melting points of the following hydrides follow in order.
Text Solution
|