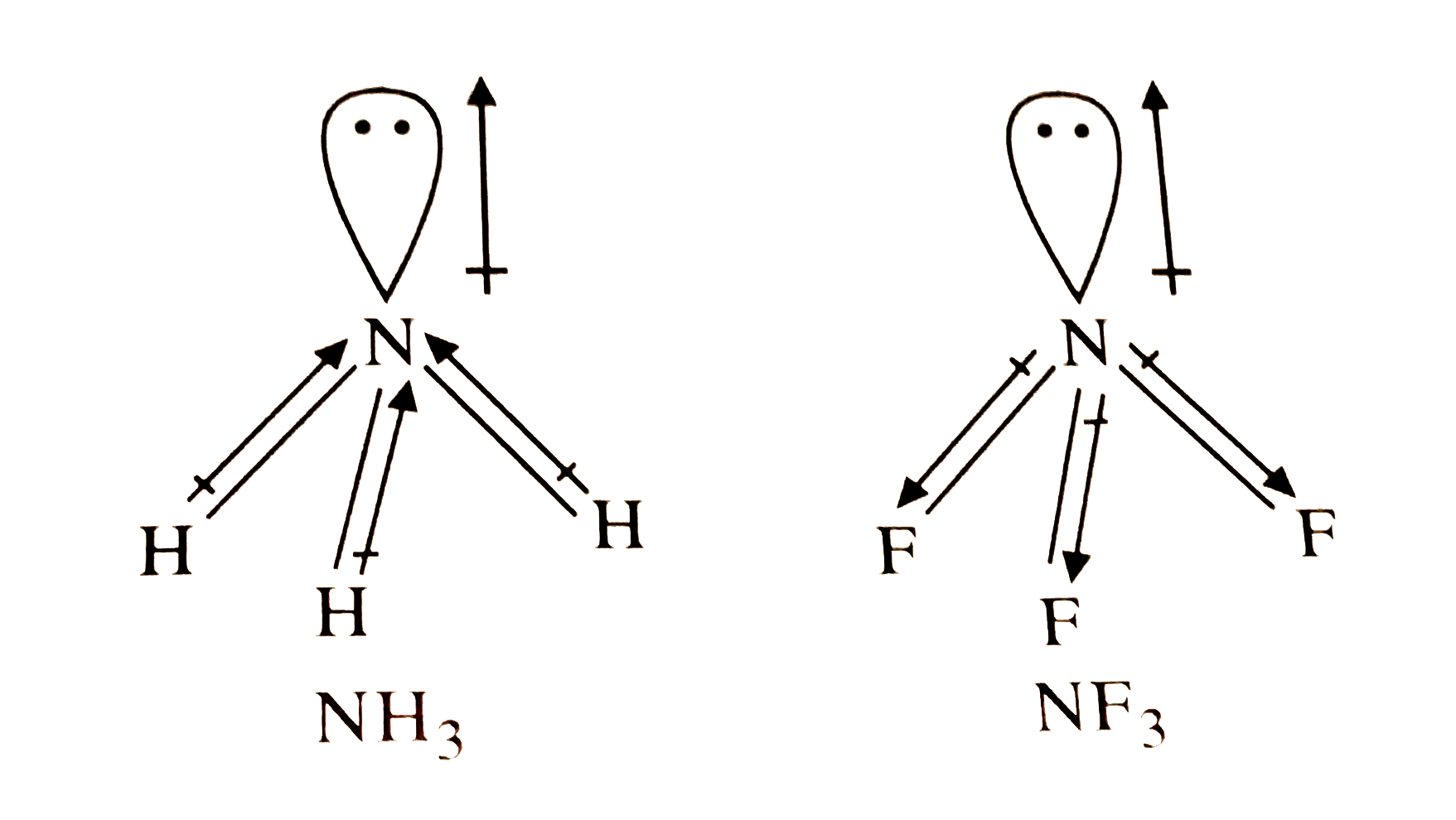A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Selected straight objective type|38 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Linked comprehension|3 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Ultimate|14 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Revision question
- Aqueous sodium hydroxide reacts with white phosphorus to form phosphin...
Text Solution
|
- The stability of the hydrides follows the order
Text Solution
|
- Which one of the following arrangements of molecules is correct on the...
Text Solution
|
- Ionic radii (in Ã…) of As^(3+), Sb^((3+) and Bi^(3+) follow the order
Text Solution
|
- The trade name of sodium hexametaphosphate is.
Text Solution
|
- Which one of the following is an oxyacid ?
Text Solution
|
- The explanation for the presence of three unpaired electrons in the ni...
Text Solution
|
- When on excess of chlorine is treated with ammonia ,the products forme...
Text Solution
|
- The three important oxidation states of phosphorus are
Text Solution
|
- Of the following compounds, the most acidic is
Text Solution
|
- Which one of the following elements is most metallic?
Text Solution
|
- Number of sigma bonds in P4 O(10) is :
Text Solution
|
- In NO3^- ion, the number of bond pair and lone pair of electrons no N-...
Text Solution
|
- In the following reaction PCl5 overset(H2 O) to HCl + A
Text Solution
|
- PH3, the hydride of phosphorus is
Text Solution
|
- The correct sequence of decrease in the bond angles of the following h...
Text Solution
|
- Which of the following is metaphosphoric acid?
Text Solution
|
- Which of the following oxyacids of phosphorus is a reducing agent and ...
Text Solution
|
- Which of the following compound is tribasic acid?
Text Solution
|
- What is the hybridisation state of the central atom in the conjugate b...
Text Solution
|