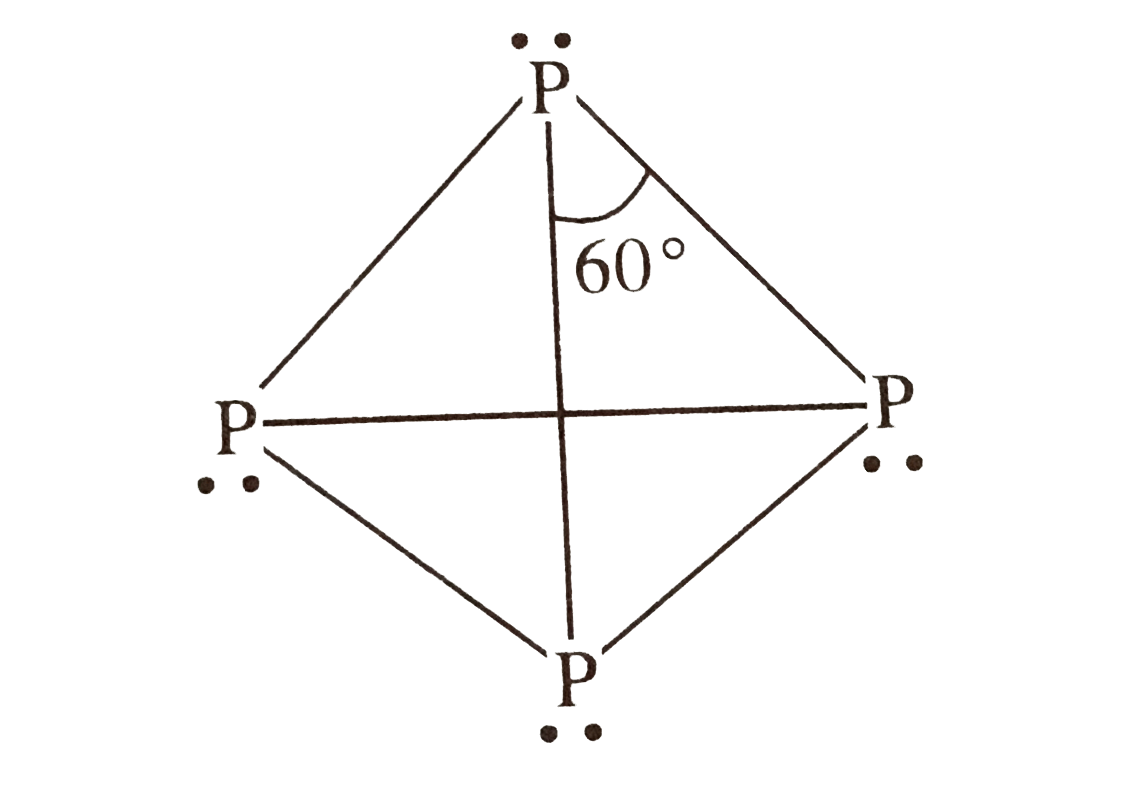
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Linked comprehension|3 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Matrix|2 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Revision question|165 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Selected straight objective type
- Yellow phosphorus does not glow in (all gases at one atmospheric press...
Text Solution
|
- Nitrozen (i) oxide is produced by
Text Solution
|
- White phosphorus (P4) has
Text Solution
|
- When phosphorus reacts with caustic soda, the products are PH(3) and N...
Text Solution
|
- Which of the following does not give NO2 on heating?
Text Solution
|
- A gas that cannot be collected over water is.
Text Solution
|
- Bonds present in N2 O5 are.
Text Solution
|
- The electronegativity of the following elements increases in the order
Text Solution
|
- Which of the following oxide of nitrogen is a coloured gas?
Text Solution
|
- Among the trihalides of nitrogen, which is the least basic ?
Text Solution
|
- The lightning bolts in the atmosphere causes the formation of nitric o...
Text Solution
|
- Concentrated HNO(3) reacts with iodine to give:
Text Solution
|
- Which one of the following is the strongest base ?
Text Solution
|
- The oxidation state of phosphorus in Ba(H2 PO2)2 is
Text Solution
|
- Amongst the following elements whose electronic configuration are give...
Text Solution
|
- Nitrogen is liberated by the thermal decomposition of only
Text Solution
|
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- Among the following species, identify the isostuctural pairs NF(3). ...
Text Solution
|
- In nitroprusside ion, the iron and NO exist as Fe(II) and NO^(+) rathe...
Text Solution
|
- On heating ammonium dichromate, the gas evolved is :
Text Solution
|