A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Linked comprehension|3 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Matrix|2 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Revision question|165 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise ULTIMATE PREPARATORY PACKAGE|16 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Selected straight objective type
- Concentrated HNO(3) reacts with iodine to give:
Text Solution
|
- Which one of the following is the strongest base ?
Text Solution
|
- The oxidation state of phosphorus in Ba(H2 PO2)2 is
Text Solution
|
- Amongst the following elements whose electronic configuration are give...
Text Solution
|
- Nitrogen is liberated by the thermal decomposition of only
Text Solution
|
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- Among the following species, identify the isostuctural pairs NF(3). ...
Text Solution
|
- In nitroprusside ion, the iron and NO exist as Fe(II) and NO^(+) rathe...
Text Solution
|
- On heating ammonium dichromate, the gas evolved is :
Text Solution
|
- One mole of calcium phosphine on reaction with excess of water gives
Text Solution
|
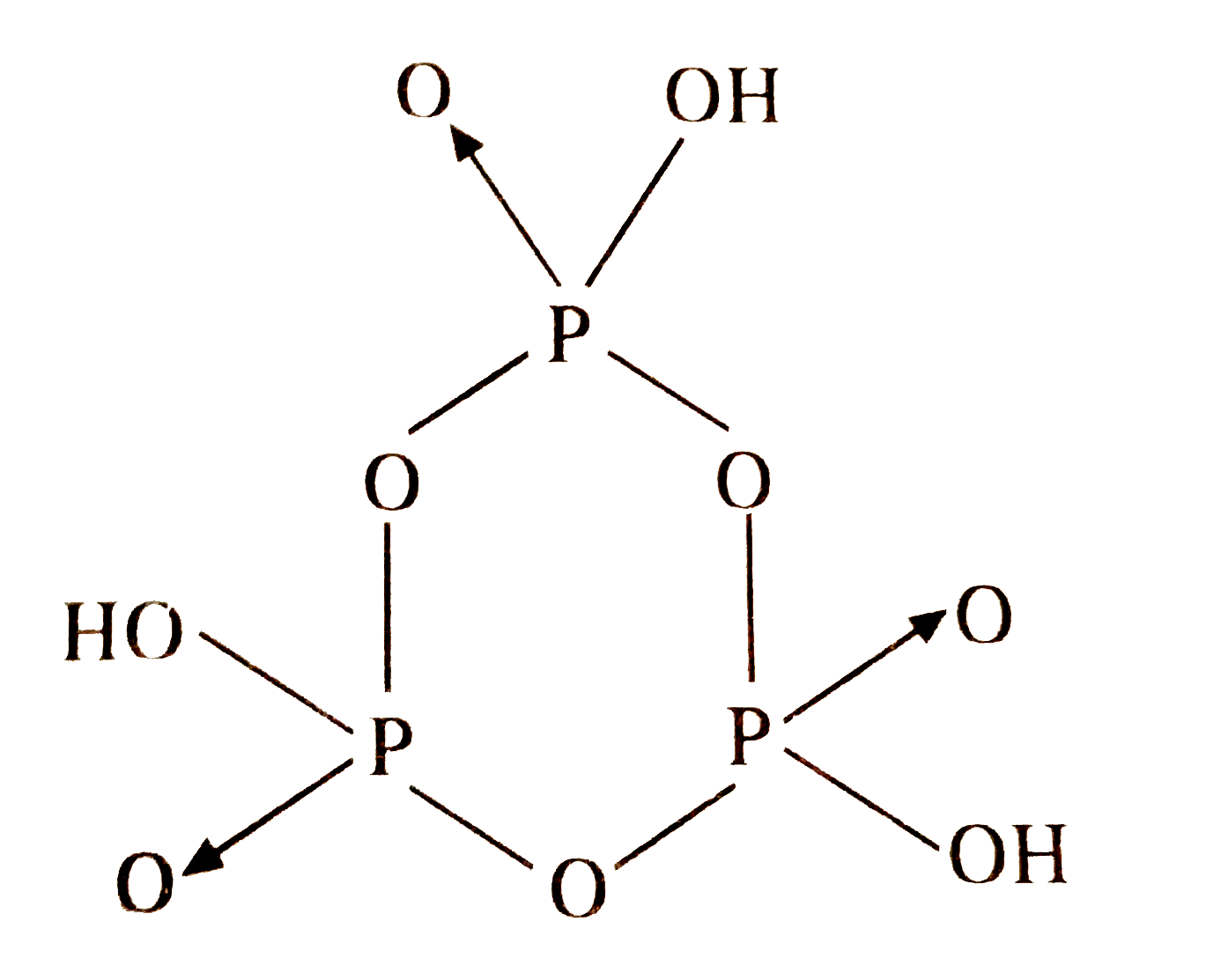
- The number of P-O-P bonds in cyclic metaphosphoric acid is.
Text Solution
|
- Ammonia can be dried by :
Text Solution
|
- The hybridisation of atomic orbitals of nitrogen in NO2^+ , NO3^- and...
Text Solution
|
- For H3 PO3 and H3 PO4, the correct choice is
Text Solution
|
- Which is the most thermodynamically stable allotropic form of phosphor...
Text Solution
|
- Which blue liquid is obtained on reacting equimolar amounts of two gas...
Text Solution
|
- The compound which has molecular nature in gas phase but ionic in soli...
Text Solution
|
- Which of the following has the least bond angle?
Text Solution
|
- The percentage of p-character in the orbitals forming p-p bonds in P4 ...
Text Solution
|
- The correct order of increasing bond angles in the following triatomic...
Text Solution
|