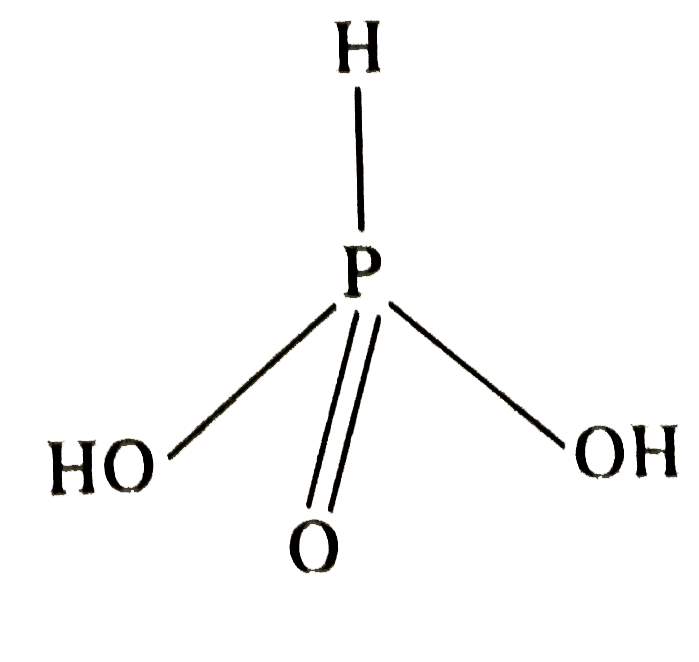A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Assertion and reason
- Assertion : H(3)PO(2) is a diabasic acid. Reason: There are two H at...
Text Solution
|
- Assertion : H(3)PO(3) is a diabasic acid. Reason: There are two H at...
Text Solution
|
- Assertion : PCl(5) is covalent in gaseous and liquid state but ionic i...
Text Solution
|
- Assertion : white phosphorus is more reactive than red phosphorus Re...
Text Solution
|
- Assertion : HNO(3) is a stronger acid than HNO(2) Reason: In HNO(3),...
Text Solution
|
- Assertion : PF(3) behaves as a lewis acid. Reason: PF(3) has a pyram...
Text Solution
|
- Assertion : White phosphorus is stored under water. Reason: White ph...
Text Solution
|
- Assertion : P(4) is more reactive than N(2) Reason: P-P single bond ...
Text Solution
|
- Assertion : Among the hydrides of N-family , NH(3) has highest boiling...
Text Solution
|
- Assertion : NH(3) is less basic than PH(3) Reason: Nitrogen is more ...
Text Solution
|
- Assertion : Between SiCl(4) and C Cl(4) only SiCl(4) reacts with water...
Text Solution
|
- Assertion : Nitrogen is less reactive than molecular oxygen. Reason:...
Text Solution
|