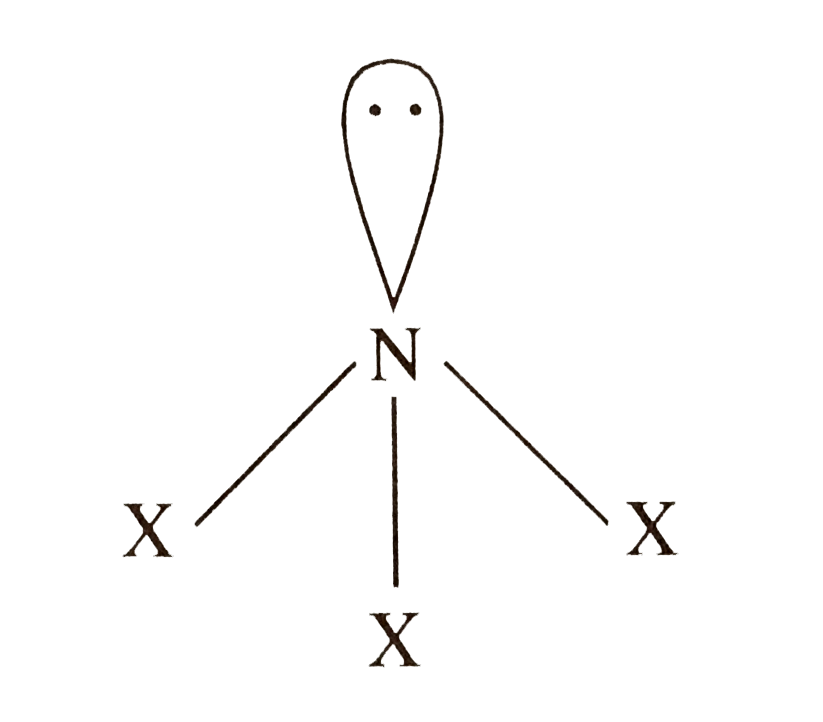
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NITROGEN FAMILY-Ultimate
- Ordinary phosphine is spontaneously inflammable due to the presence of
Text Solution
|
- The trihalide of nitrogen with highest dipole moment is
Text Solution
|
- In the compounds of the type POX3, P atoms show multiple bonding of th...
Text Solution
|
- SbF(3) a fluorinating agent for non-metal compounds is called
Text Solution
|
- Which of the following does not exist ?
Text Solution
|
- Which of the following has no basic properties ?
Text Solution
|
- In the reaction 2Ca(3)[PO(4)](2)+6SiO(2) overset(Delta)to6CaSiO(3) ...
Text Solution
|
- Which of the following is not soluble in excess of water ?
Text Solution
|
- From a mixture of yellow P and red P, yellow P can be removed by
Text Solution
|
- Na(2)HPO(4) solution in water is
Text Solution
|
- The most stable pentaoxide is
Text Solution
|
- In N(2)O(5) valence of nitrogen is
Text Solution
|
- Some KH(2)PO(2) is dissolved in D(2)O . The resulting solution is drie...
Text Solution
|
- Doping Si or Ge with As or P will produce
Text Solution
|