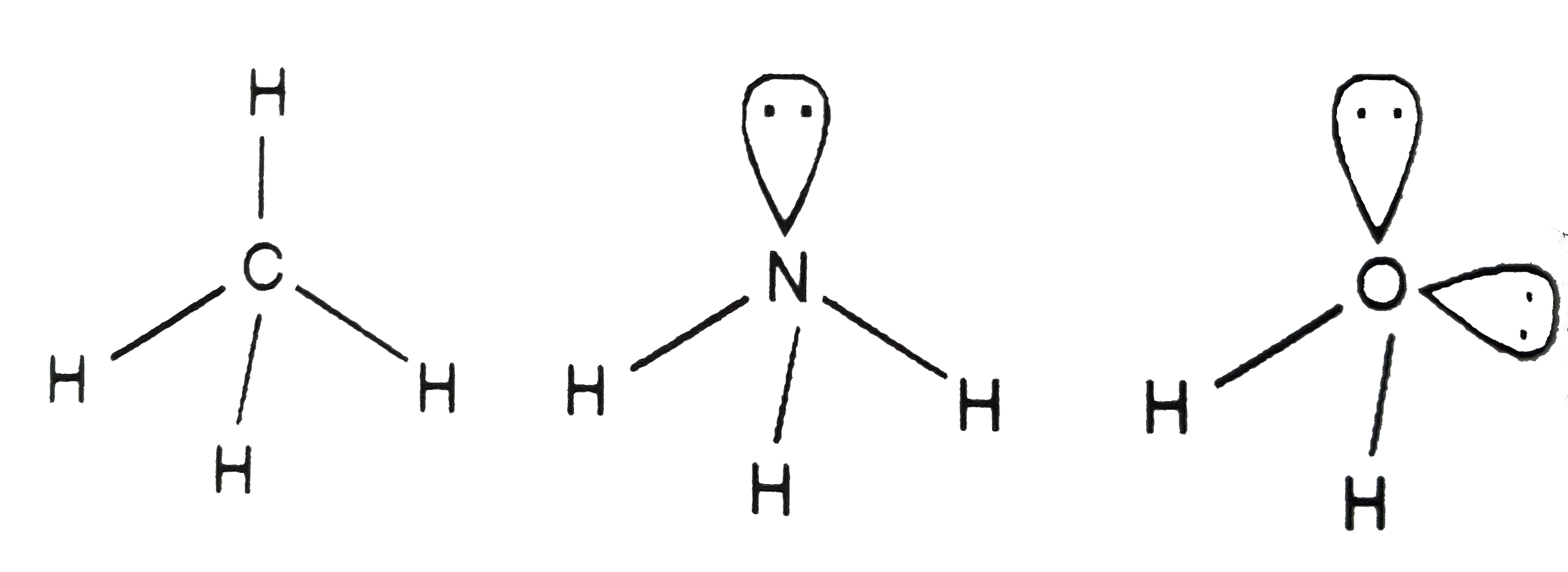
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE OXYGEN FAMILY
DINESH PUBLICATION|Exercise Selected Straight objective|34 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise MATRIX MATCH|2 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise PREPARATORY PACKAGE|14 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 VideosTHE P-BLOCK ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Choice Questions (McQs)|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE OXYGEN FAMILY-REVISION
- iodine oxidises the S2O3^(2-) ion to
Text Solution
|
- Which of the following has the highest dipole moment ?
Text Solution
|
- Which of the following has the least bond angle ?
Text Solution
|
- Ozone is not
Text Solution
|
- Which liberates SO2 with dil. H2SO4?
Text Solution
|
- The number of unpaired electrons in the p-subshell of oxygen atom
Text Solution
|
- Which would quickly absorb oxygen?
Text Solution
|
- Oleum is
Text Solution
|
- Sulphur molecule is
Text Solution
|
- Which of the following formula represents the fuming sulphuric acid (o...
Text Solution
|
- When sulphur is boiled with Na(2)SO(3) solution, the compound formed i...
Text Solution
|
- Ozone belong to which group of the periodic table ?
Text Solution
|
- Oxygen is more electronegative than sulphur. Yet H2S is acidic while H...
Text Solution
|
- Polyanion formation is maximum in
Text Solution
|
- Which one of the following property is not correct for ozone?
Text Solution
|
- All of the following decompose easily on heating to give O2 except
Text Solution
|
- Sometimes yellow turbidity appears while passing H(2)S gas even in the...
Text Solution
|
- K2CS3 can be called potassium
Text Solution
|
- Sulphuric acid has great affinity for water because it
Text Solution
|
- Heavy water is manufactured by :
Text Solution
|