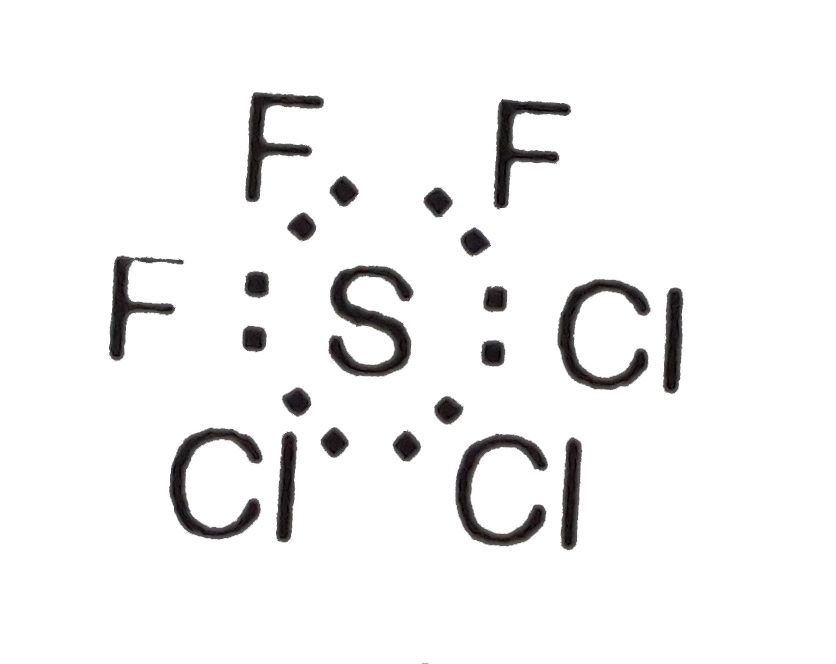A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE OXYGEN FAMILY
DINESH PUBLICATION|Exercise Selected Straight objective|34 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise MATRIX MATCH|2 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise PREPARATORY PACKAGE|14 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 VideosTHE P-BLOCK ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Choice Questions (McQs)|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE OXYGEN FAMILY-REVISION
- When potassium ferrocyanide crystals are heated with concentrated sulp...
Text Solution
|
- The number of electrons that are paired in oxygen molecule is
Text Solution
|
- The shape of the molecule SF2Cl2 is
Text Solution
|
- The correct order of O-O bond length in O2,H2 O and O3.
Text Solution
|
- Which of the following has the largest ionic size?
Text Solution
|
- Which of the following isoelectronic ions has the lowest ionization en...
Text Solution
|
- States of hybridization of P in PF5 and S in SF6 are respectively?
Text Solution
|
- Atomicity of sulphur in rhombic sulphur is
Text Solution
|
- Which of the following is not tetrahedral ?
Text Solution
|
- There is S-S bond in
Text Solution
|
- The compound of sulphur that can be used as refrigerant is
Text Solution
|
- Which of the following causes damage to the building containing calciu...
Text Solution
|
- Which of the following is not linear ?
Text Solution
|
- Number of bonds in SO2 are
Text Solution
|
- Oxidation state of oxygen is zero in
Text Solution
|
- Which of the following does not exhibit sp^3- hybridisation ?
Text Solution
|
- The reaction of HCOOH with conc. H2 SO4 gives :
Text Solution
|
- Which one of the following is an oxyacid ?
Text Solution
|
- The products obtained by passing chlorine through hypo solution are
Text Solution
|
- Which of the following species is basic and reducing ?
Text Solution
|