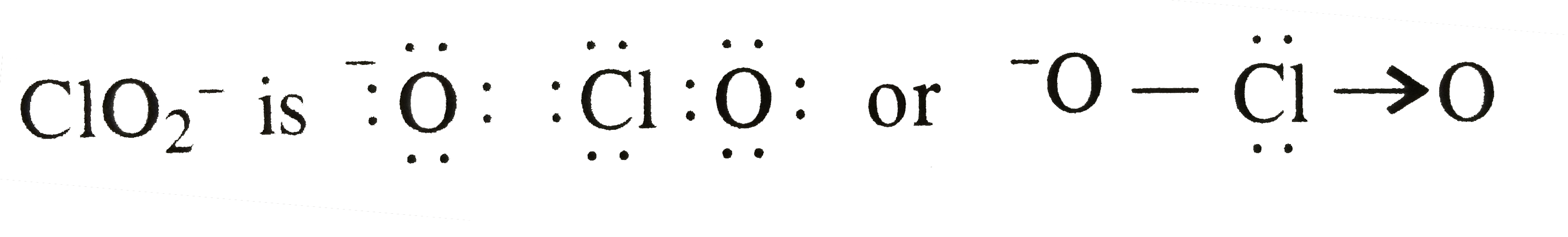A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE HALOGEN FAMILY
DINESH PUBLICATION|Exercise MATRIX MATCH|2 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise INTEGER|4 VideosTHE HALOGEN FAMILY
DINESH PUBLICATION|Exercise REVISION|194 VideosTHE CARBON FAMILY
DINESH PUBLICATION|Exercise Ultimate|11 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Ultimate|14 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE HALOGEN FAMILY-Selected Straight Objective Type MCQs
- Concentrated HNO(3) reacts with iodine to give:
Text Solution
|
- Which of the following is the strongest acid?
Text Solution
|
- The elements which exists in the liquid state is/ are
Text Solution
|
- Increasing order of ionic size : N^(3-),Na^(+),F^(-),O^(2-),Mg^(2+)
Text Solution
|
- The halogen which is most easily reduced is
Text Solution
|
- The type of hybrid orbitals used by chlorine atom in ClO(2)^(-) is :
Text Solution
|
- Arrange the acids (I) H2SO3, (II) H3PO3 and (III) HClO3 in the decreas...
Text Solution
|
- The following acid have arrange in the order of decreasing strength. I...
Text Solution
|
- KF combines with to form KHF2. The compound contains the species :
Text Solution
|
- Which one of the following are pseudohalide ions ?
Text Solution
|
- The correct order of acidic strength is
Text Solution
|
- The set with the correct order of acidity is
Text Solution
|
- The reaction 3ClO^(-)(aq)rarrClO(3)^(-)(aq)+2Cl^(-)(aq) an example of ...
Text Solution
|
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- In which of the following molecules /ions , are all the bonds not equa...
Text Solution
|
- What products are expected from the desproprtionation reactin of hypoc...
Text Solution
|
- Which one of the following orders is not correct in accordance with t...
Text Solution
|
- In which of the following molecules all the bonds are not equal ?
Text Solution
|
- Which one of the following ionic species has the greatest proton affin...
Text Solution
|
- Identify the incorrect statement amongst the following
Text Solution
|