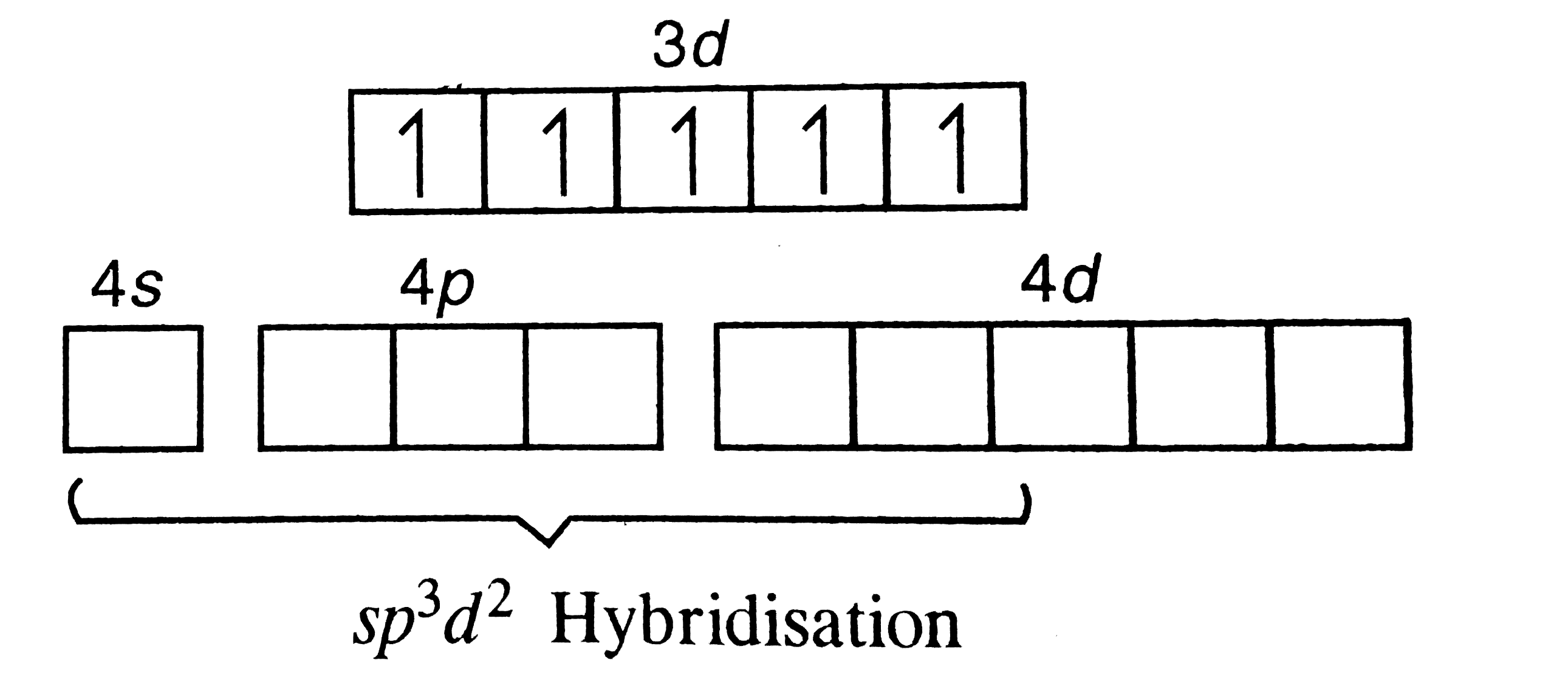
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise REVISION QUESTIONS|165 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|84 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -Brain storming multiple choice question
- Hexafluorocbaltate (III) ion is an outer orbital complex. The number o...
Text Solution
|
- When excess of ammonia is added to CuSO4 solution, the deep blue colou...
Text Solution
|
- Select the correct statements(s) :
Text Solution
|
- A compound has the empirical formula CoCl(3).5NH3. When an aqueous sol...
Text Solution
|
- In the above question
Text Solution
|
- Which is true for the complex [Ni(en)2]^(2+) ?
Text Solution
|
- The isomerism exhibited by the co-ordination complex [Cr(NH3)2(H(2)O)(...
Text Solution
|
- The pair in which both species have same magnetic moment (spin only va...
Text Solution
|
- The ligands in anti- cancer drug cisplatin are
Text Solution
|
- Which of the following is not correct ?
Text Solution
|
- The two complexes given below are
Text Solution
|
- Ammonia forms the complex [Cu(NH3)4]^(2+) with copper ions in alkaline...
Text Solution
|
- The complex [Fe(H(2)O)(5)NO]^(2+) is formed in the brown ring test for...
Text Solution
|