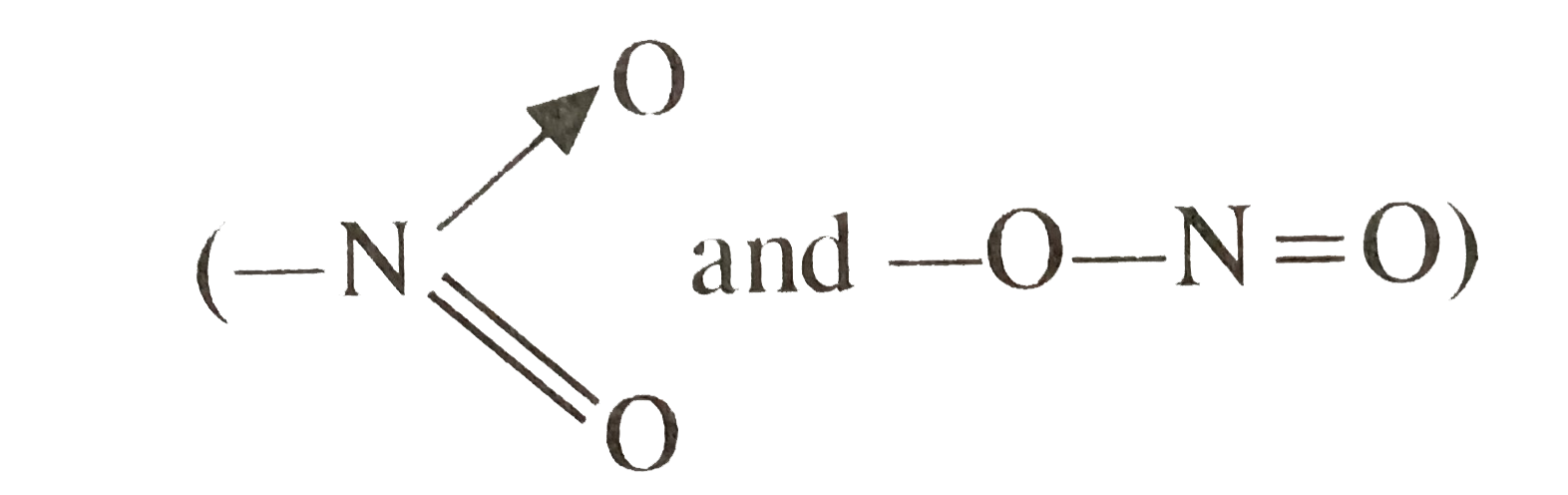A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise SELECTED STRAIGHT OBJECTIVE TYPE MCQS|84 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise COMPREHENNSION TYPE MCQS|5 VideosCO-ORDINATION COMPOUNDS
DINESH PUBLICATION|Exercise Brain storming multiple choice question|12 VideosCHEMISTRY IN EVERY DAY LIFE
DINESH PUBLICATION|Exercise Unit test-9|50 VideosCYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINES
DINESH PUBLICATION|Exercise Unit test - 8|20 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CO-ORDINATION COMPOUNDS -REVISION QUESTIONS
- The coordination number of Fe (II) in oxyhaemoglobin is
Text Solution
|
- In[Co(NH(3))(6)]Cl(3),the number of covalent bonds is
Text Solution
|
- Which of the following compounds exhibits linkage isomerism?
Text Solution
|
- The oxidation number of cobalt in K [Co(CO)(4)] is
Text Solution
|
- The IUPAC name of K(3)[Ir(C(2)O(4))(3)] is
Text Solution
|
- Chemical formula for in iron(III) hexacyanoferrate(II) is
Text Solution
|
- The effective atomic number of iron in [Fe(CN)(6)]^(3-) is
Text Solution
|
- The geometry of the compound [Pt(NH(3))(2)Cl(2)] is
Text Solution
|
- Which of the following cannot show linkage isomerism?
Text Solution
|
- IUPAC name of K(3)[Fe(CN)(6)] is
Text Solution
|
- The shape of cuprammonium ion is
Text Solution
|
- Which of the following species represent the example of dsp^(2)-hybrid...
Text Solution
|
- Consider the following complex [Co(NH(3))(5)CO(3)]ClO(4) The coordin...
Text Solution
|
- In [Co(C(2)O(4))(3)]^(3-), the isomerism shown is .
Text Solution
|
- In the complex [Fe(H(2)O)(6)]^(3+) [Fe(CN)(6)]^(3-) [Fe(C(2)O(4))(3)]^...
Text Solution
|
- The colour of CoCl(3).5NH(3).H(2)O is
Text Solution
|
- Atomic numbers of Cr and Fe are respectively 24 and 26. Which of the f...
Text Solution
|
- The hypothetical complex triamminediaquachloridocobalt(III) chloride c...
Text Solution
|
- In the silver plating of copper, K[Ag(CN)(2)] is used instead of AgNO(...
Text Solution
|
- Both geometrical and optical isomerism are shown by
Text Solution
|